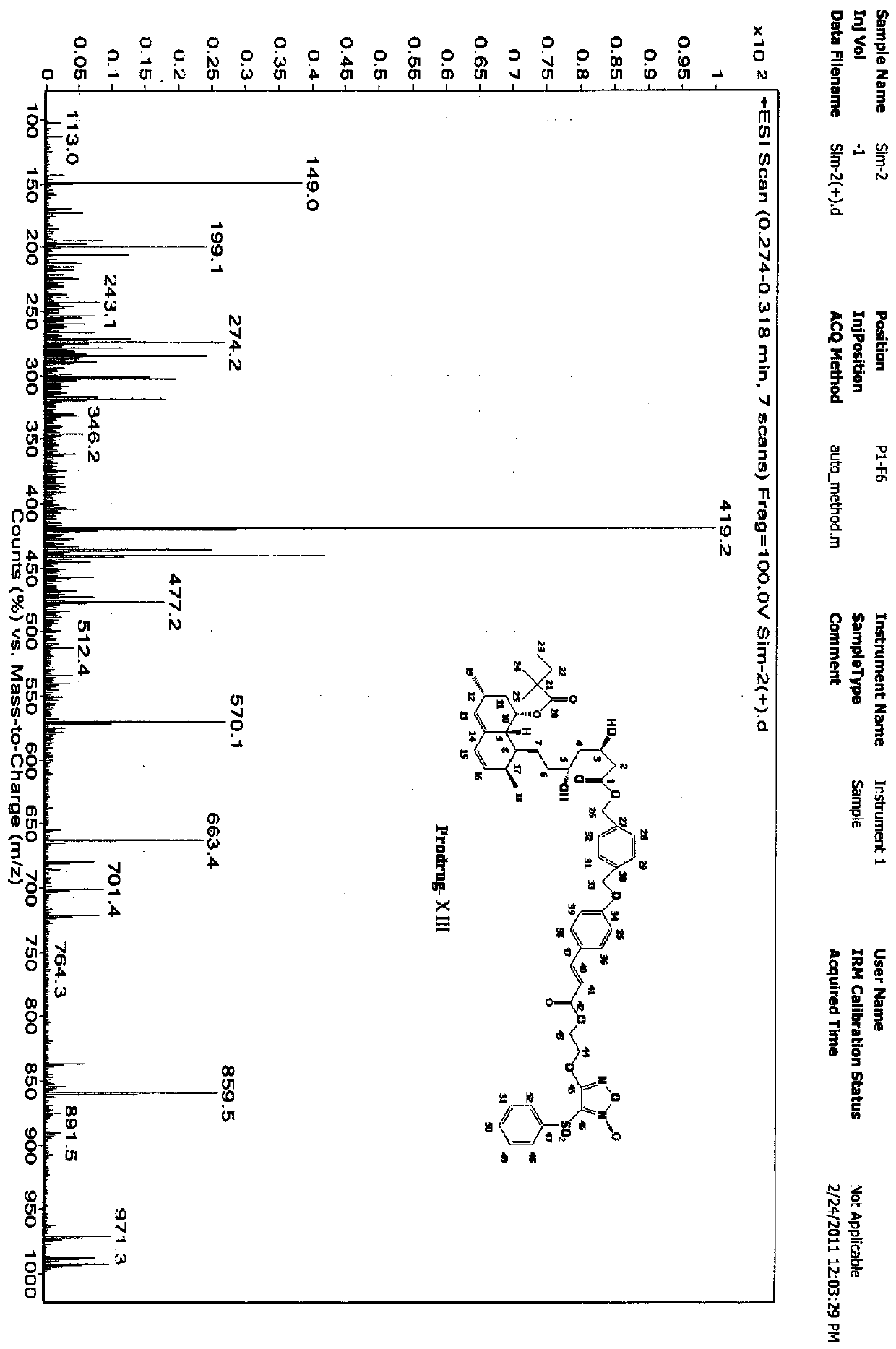
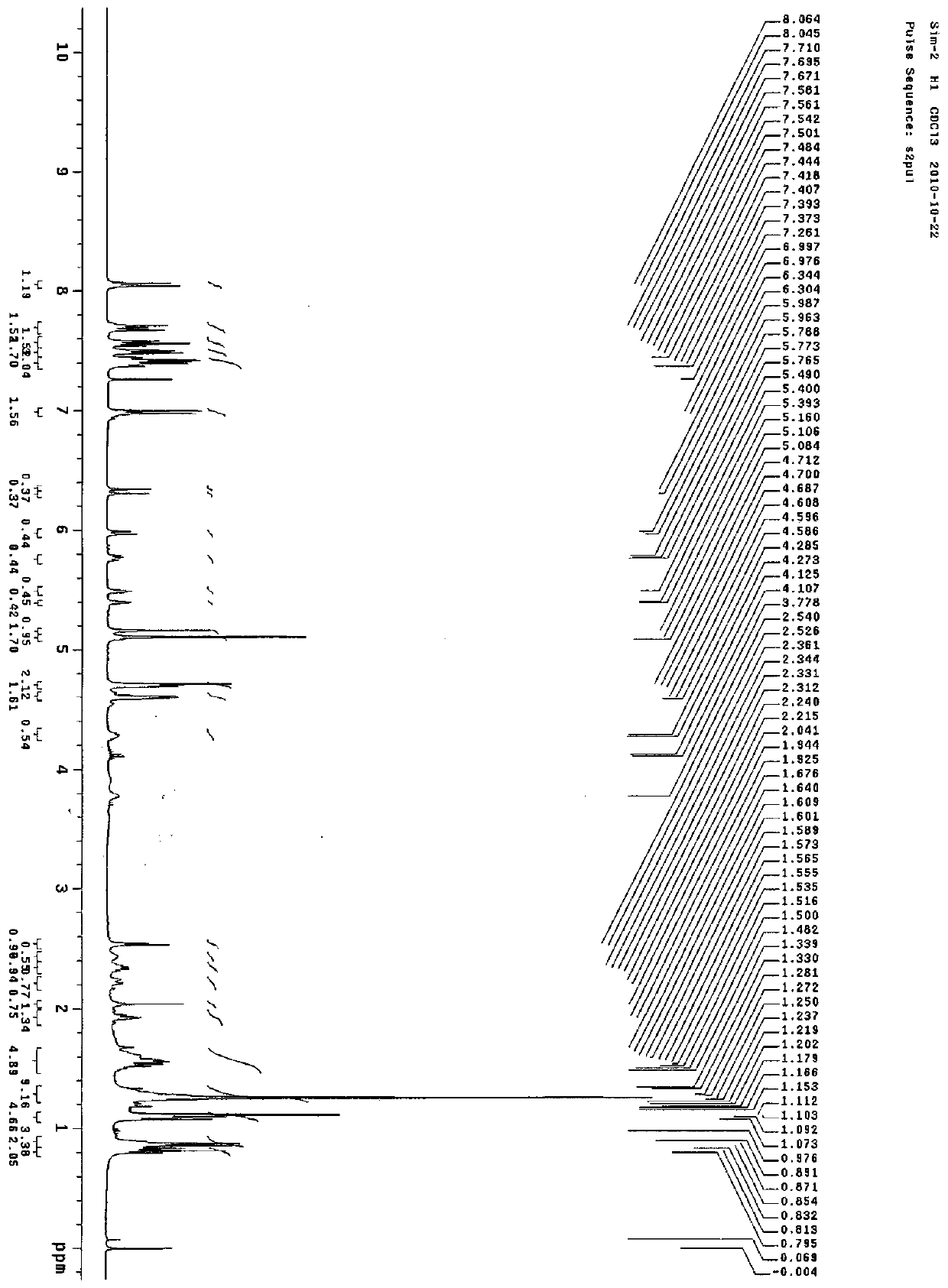
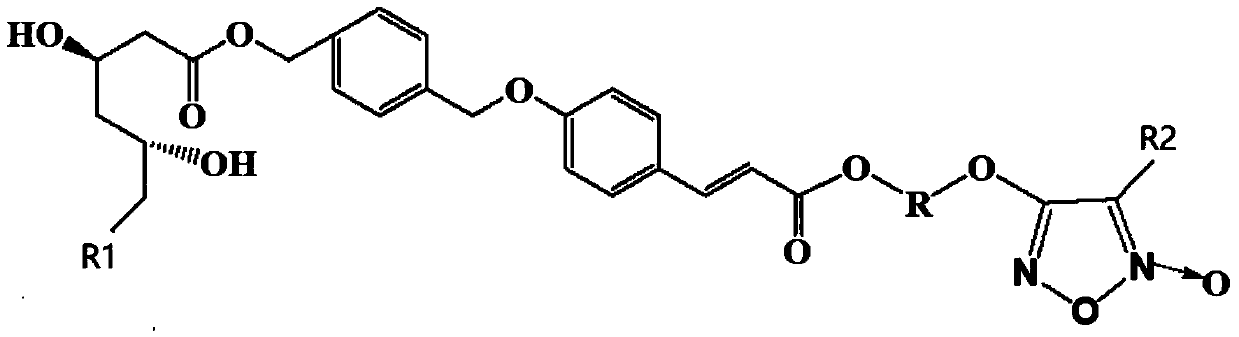
Furoxan NO donor-type statin derivative and preparation method thereof
A technology of derivatives and statins, which is applied in the field of medicinal chemical synthesis, can solve the problems of few research reports, achieve the effect of enhancing curative effect, good therapeutic effect, and avoiding the effect of mismatching mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of benzenesulfonyl substituted furazan nitrogen oxides
[0059] 1.1 Preparation of 2-phenylthioacetic acid (9):
[0060] Take 99.8g (100ml, 0.98mol) of thiophenol, dissolve 39.2g (0.98mol) of NaOH in 500ml of 95% EtOH, and after the NaOH dissolves completely drop to room temperature, add dropwise 101g (1.07mol) of chloroacetic acid and NaOH 2 CO 3 500ml of the aqueous solution prepared by 56.7g (0.535mol) was added, stirred at room temperature for 3h after the dropwise addition, and then refluxed for 1h. Reduce to room temperature and use 6N HCl to adjust pH = 2, evaporate EtOH under reduced pressure, stir overnight at room temperature, add 1 L of water to the system, stir for 1 h, a large amount of white solid appears, filter, and dry the sample to obtain 9164 g of white solid (mp=63.3- 64.5°C, literature value mp=60-62°C).
[0061] 1.2 Preparation of 3,4-diphenylsulfonyl-1,2,5-oxadiazole-2-oxide (11):
[0062]Dissolve 164g (0.98mol) of compound 9 in gl...
Embodiment 2
[0089] Preparation of Phenyl Substituted Furazan Oxides
[0090] 2.1 Preparation of 3-hydroxymethyl-4-phenyl-1,2,5-oxadiazole-2-oxide (25):
[0091]Take 10g (0.075mol) of cinnamyl alcohol and dissolve it in 120ml of glacial acetic acid, stir mechanically, after the cinnamyl alcohol is dissolved, add 15.5g (0.22mol) of sodium nitrite, react for 2h, add 200ml of water to the reaction solution to terminate the reaction, EA 500ml *2 Extraction, washing the EA layer with 100ml saturated brine, concentrating the EA layer, column chromatography [PE / EA=2:1 (v:v) elution], after post-treatment, 259.8g of yellow oily substance was obtained. .
[0092] 2.2 3-[(4-Methoxyformyloxy)phenyl]-2-propenoic acid (4-phenyl-1,2,5-oxadiazole-2-oxide-3)-methyl ester (26) Preparation of:
[0093] Take 6.0g (27mmol) of compound 15, dissolve it in 60ml of pyridine, add 4.9ml (37.8mmol, 7.8mmol / ml) of benzenesulfonyl chloride dropwise in an ice-salt bath, react at room temperature for 30min, and add 2...
Embodiment 3
[0099] Preparation of intermediate 4-coumaric acid derivatives
[0100] 3.1 Preparation of E-4-hydroxy-phenylacrylic acid-4'-bromobutyl ester (4):
[0101] Take 4.9g of 4-coumaric acid (FW164, 30mmol) and dissolve it in 200ml of acetone. After dissolving, add 24ml of triethylamine and 12ml of 1,4-dibromobutane (100mmol), and reflux for 6h (60°C). After filtration, the filtrate was concentrated to an oily substance, and column chromatography [PE / EA=3:1 (v:v) elution] was performed to obtain 4.8 g of white solid 4.
[0102] 3.2 Preparation of E-4-hydroxy-phenylacrylate-4'-(nitrooxy)butyl ester (5):
[0103] Dissolve 4.8g (16mmol) of compound 5 in 100ml acetonitrile, add AgNO 3 6.9g (41mmol), reflux reaction for 5h (85°C), lowered to room temperature, filtered, the filtrate was concentrated to oily matter, column chromatography [PE / EA=3:1 (v:v) elution], after post-treatment to obtain Crude 5 11.2 g.
[0104] 3.3 Preparation of E-4-(2-chloromethyl)benzyloxyphenylacrylate-4'-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com