Benzenesulfonyl furazan modified gemcitabine derivative and preparation method and use thereof
A technology of benzenesulfonylfurazan and gemcitabine, applied in the field of gemcitabine derivatives modified by benzenesulfonylfurazan and its preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
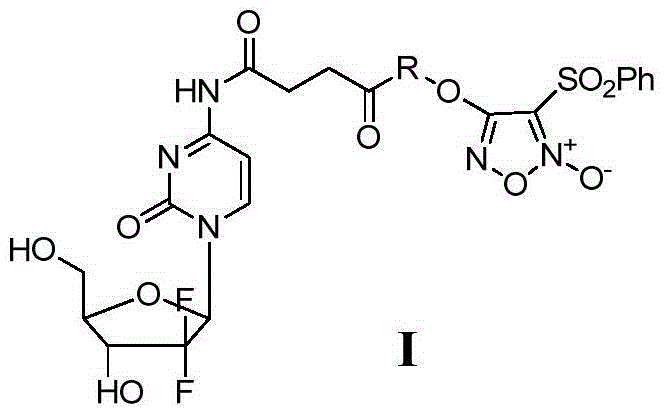
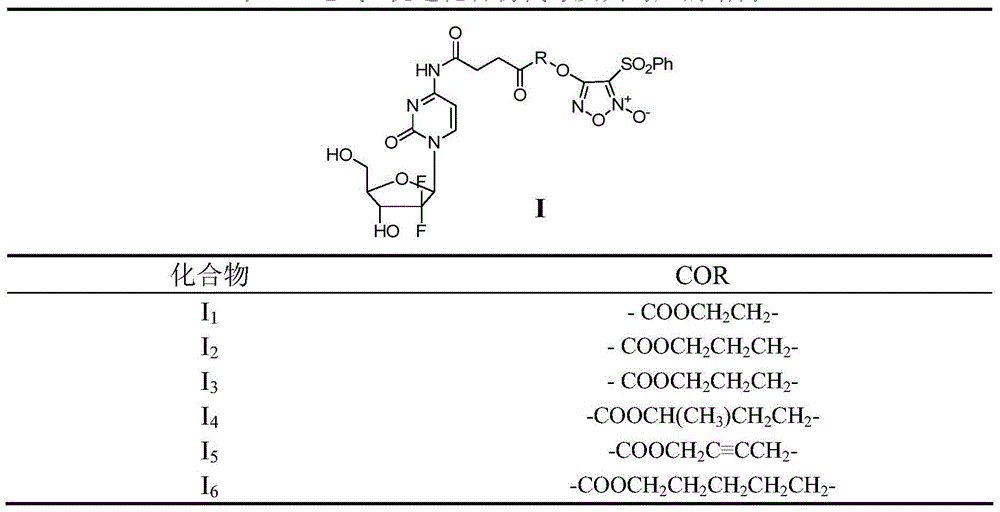
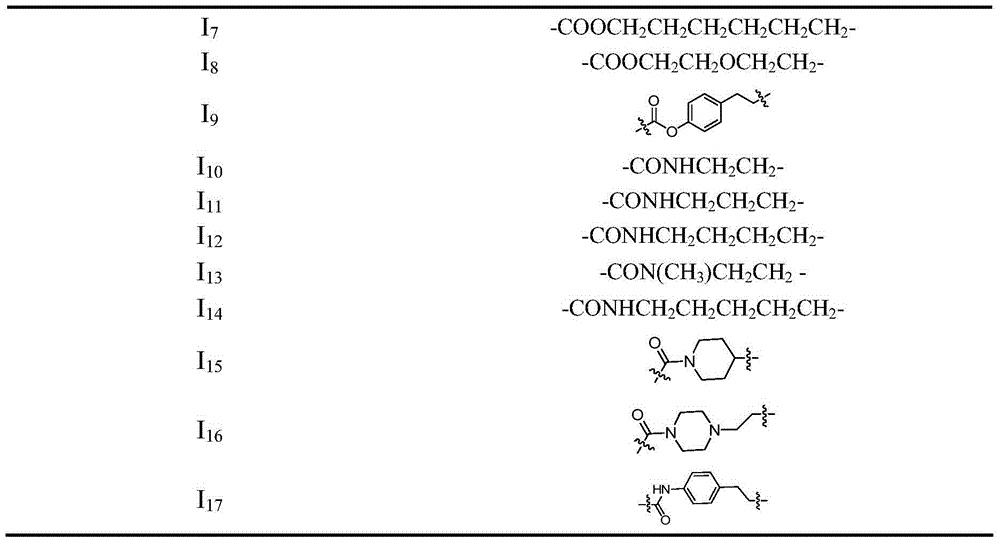
[0050] Example 1 4-(2-((4-((1-((2R,4R,5R)-3,3-difluoro-4-hydroxyl-5-(hydroxymethyl)tetrahydrofuran-2-yl)- 2-oxo-1,2-dihydropyrimidin-4-yl)amino)-4-oxobutyryl)oxy)ethoxy)-3-(phenylsulfonyl)-1,2,5- Oxadiazole-2-oxide (I 1 ) preparation
[0051] 2-Phenylthioglycolic acid (2)
[0052] Dissolve compound 1 (12.10g, 0.11mol) and sodium hydroxide in 50mL of 95% ethanol, add 100mL of aqueous solution made of chloroacetic acid (11.40g, 0.12mol) and sodium carbonate (6.35g, 0.06mol), at room temperature Stir for 3h, reflux for 1h. After cooling to room temperature, 6 mol / L hydrochloric acid was added to adjust the pH to 2, a white precipitate was formed, and filtered to obtain 16.40 g of white rod-shaped crystals, yield 89.0%, mp: 60-62°C.
[0053] 3,4-Diphenylsulfonyl-1,2,5-oxadiazole-2-oxide (4)
[0054] Dissolve compound 2 (16.00g, 0.10mol) in 65mL glacial acetic acid, add 30% hydrogen peroxide (20mL, 0.20mol), stir at room temperature for 2.5h to obtain intermediate compound ben...
Embodiment 2
[0066] Example 2 4-(2-((4-((1-((2R,4R,5R)-3,3-difluoro-4-hydroxyl-5-(hydroxymethyl)tetrahydrofuran-pyridin-2-yl )-2-oxo-1,2-dihydropyrimidin-4-yl)amino)-4-oxobutanoyl)oxy)propoxy)-3-(phenylsulfonyl)-1,2, 5-oxadiazole-2-oxide (I 2 ) preparation
[0067] 4-(3-Hydroxypropoxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole-2-oxide (5b)
[0068] Referring to the synthesis method of (5a), 1,3-propanediol was reacted with compound (4) instead of ethylene glycol, and a white solid (5b) was finally obtained with a yield of 57.2%.
[0069] 4-(2-((3-carboxypropyl)oxy)propoxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole-2-oxide (6b)
[0070] Referring to the synthesis method of (6a), (5b) instead of (5a) was reacted with 1,4-succinic anhydride and DMAP to finally obtain light yellow solid (6b) with a yield of 92.0%.
[0071] 4-(2-((4-((1-((2R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl) ylsilyl)oxy)methyl)-3,3-difluoro-tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)amino...
Embodiment 3
[0076] Example 3 4-(2-((4-((1-((2R,4R,5R)-3,3-difluoro-4-hydroxyl-5-(hydroxymethyl)tetrahydrofuran-pyridin-2-yl )-2-oxo-1,2-dihydropyrimidin-4-yl)amino)-4-oxobutanoyl)oxy)butoxy)-3-(phenylsulfonyl)-1,2, 5-oxadiazole-2-oxide (I 3 ) preparation
[0077] 4-(3-Hydroxybutoxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole-2-oxide (5c)
[0078] Referring to the synthesis method of (5a), 1,4-butanediol was reacted with compound (4) instead of ethylene glycol, and a white solid (5c) was finally obtained with a yield of 52.6%.
[0079] 4-(2-((3-carboxypropyl)oxy)butoxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole-2-oxide (6c)
[0080] Referring to the synthesis method of (6a), (5c) instead of (5a) was reacted with 1,4-succinic anhydride and DMAP to finally obtain light yellow solid (6b) with a yield of 88.0%.
[0081] 4-(2-((4-((1-((2R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl) ylsilyl)oxy)methyl)-3,3-difluoro-tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)amino)-4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com