Preparation and application of ergosterin derivative
A technology for ergosterol and derivatives, applied in the field of compound preparation, can solve the problems of difficult quality control, complex and changeable components and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method for separating and extracting new ergosterol derivatives ganodermasides C and D from Ganoderma lucidum spore powder of traditional Chinese medicine, the specific steps are:
[0031] 1) Crushing and extraction:
[0032] 1500g of traditional Chinese medicine Ganoderma lucidum spore powder was extracted with 10L of methanol (industrial grade) at room temperature for 5 days (with occasional shaking). After suction filtration and concentration, 111.1 g of crude methanol extract was obtained, which was equally divided into two parts for extraction, and extracted and separated twice with ethyl acetate (2500 ml) and water (2500 ml) alternately. A total of 75.7 g of crude ethyl acetate layer was obtained.
[0033] 2) Separation and purification:
[0034] The crude ethyl acetate layer was first separated by a silica gel open column (200-300 mesh, the following solvent systems by volume ratio, n-hexane:ethyl acetate=100:0, 90:10, 70:30, 50:50); Then, the sa...
Embodiment 1
[0037] Qualitative identification of the physical and chemical characteristics and chemical structure of compound 1 and compound 2 obtained in embodiment 1:
[0038] The structures of compounds 1 and 2 were confirmed by HR-MS, 1 H NMR, 13 Confirmed by C NMR, DEPT, H-HCOSY, HMBC, HSQC and HOHAHA.
[0039] Physical and chemical properties of compound 1: light yellow solid, molecular formula is C 28 h 38 o 3 ;HR ESI-TOF-MS: m / z 423.2877[M+H] + , theoretical value C 28 h 39 o 3 [M+H] + 423.2894. Infrared spectrum (KBr) value: 3500, 1678cm -1 ; Ultraviolet spectrum (solvent is methanol): the maximum absorption peak is 336nm; hydrogen spectrum and carbon spectrum data are shown in Table-1.
[0040] Physical and chemical properties of compound 2: light yellow solid, molecular formula is C 28 h 40 o 2 ;HR ESI-TOF-MS: m / z 409.3075[M+H] + , theoretical value C 28 h 41 o 2 : 409.3101. Infrared spectrum (KBr) value: 3455, 1635cm -1 ; Ultraviolet spectrum (solvent is me...
Embodiment 3
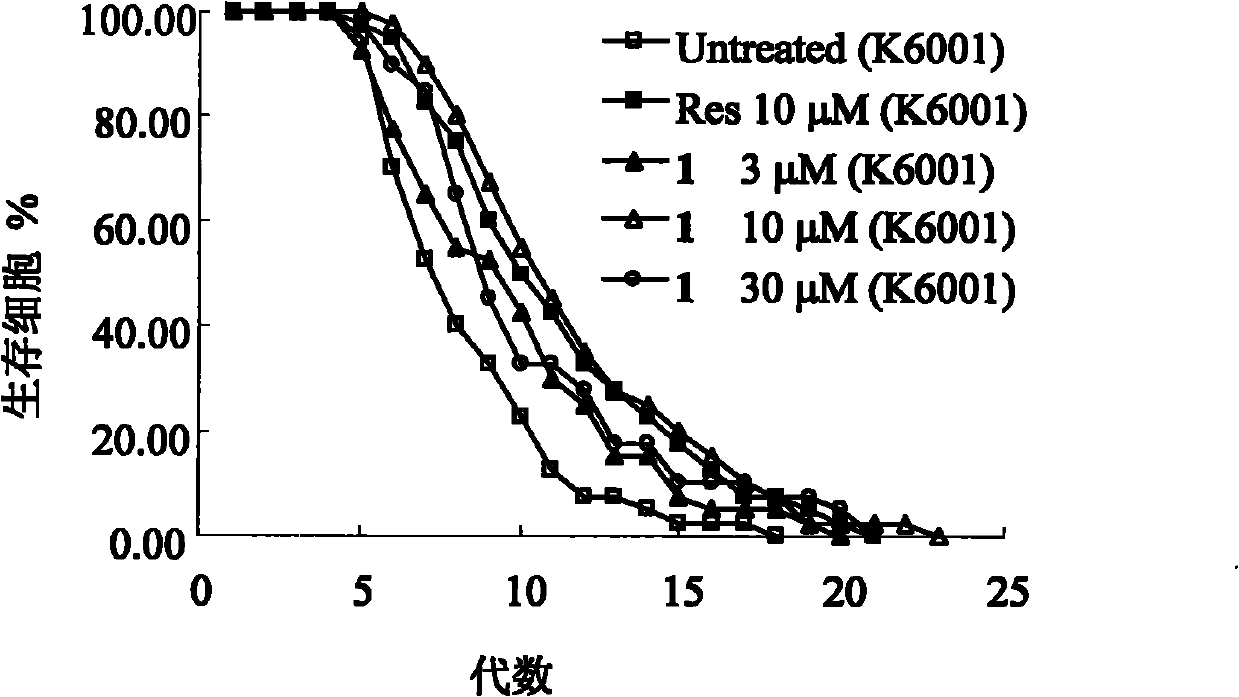
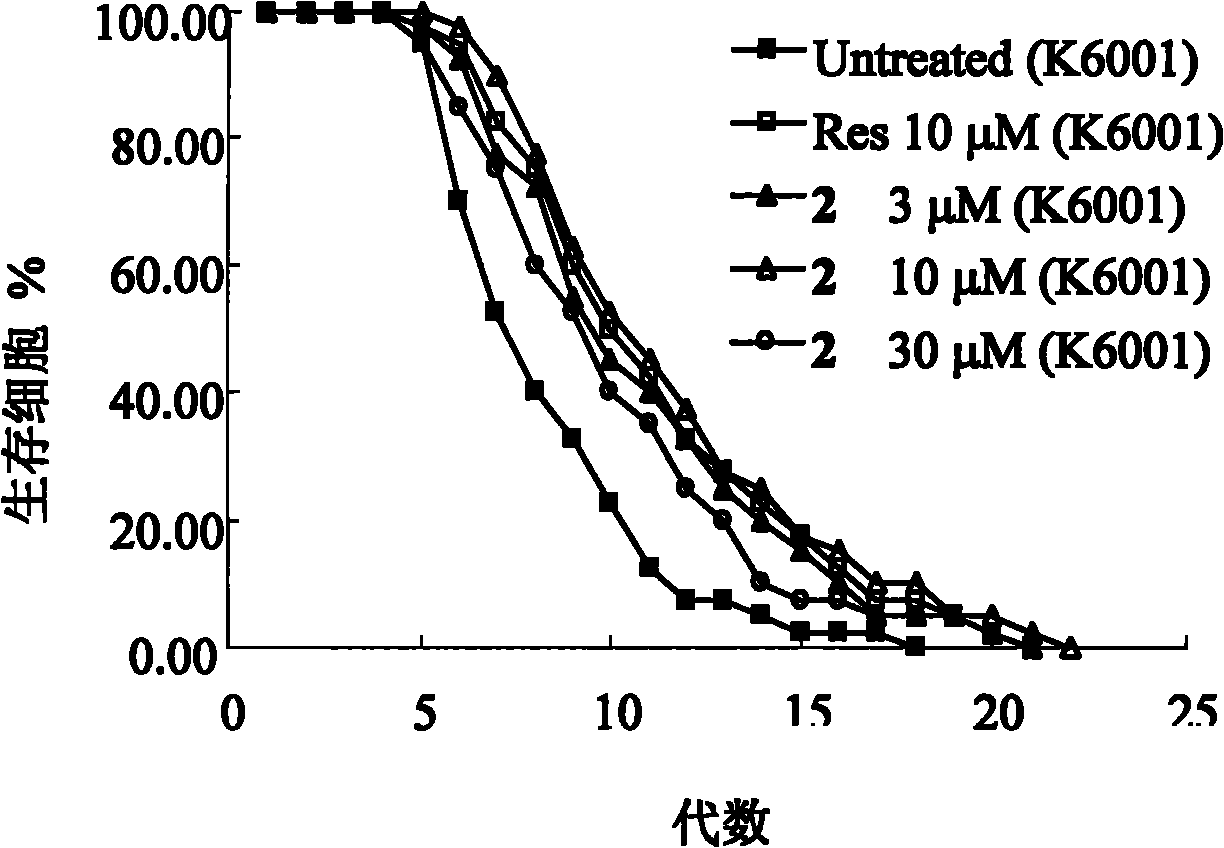
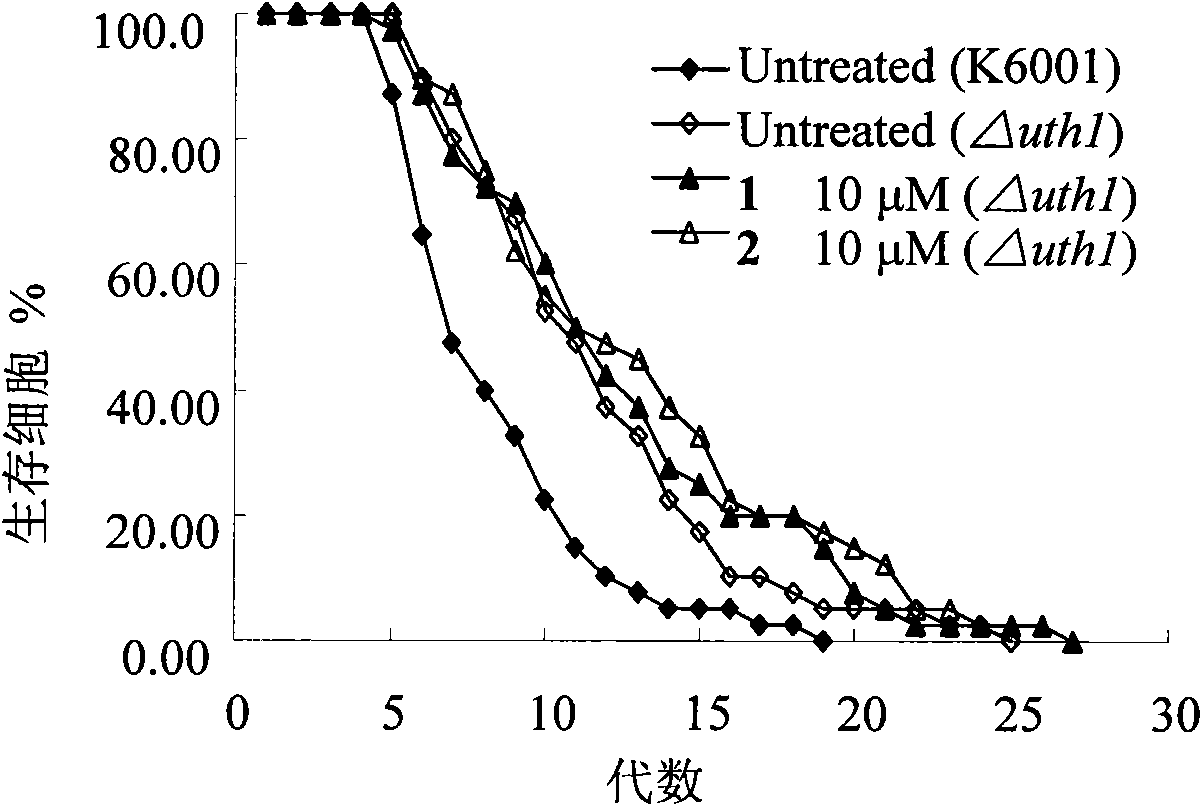
[0052] Compound 1 and Compound 2 significantly prolong the replicative lifespan of yeast cells in the in vitro screening model of anti-aging compounds.
[0053] Saccharomyces cerevisiae is a single-celled eukaryote, whose complete genome data has been obtained, and is currently a commonly used aging model organism. Its asymmetric division is often used to study its replicative lifespan, but requires the removal of daughter cells with a micromanipulator before the mother cell divides. The mutant strain of Saccharomyces cerevisiae (K6001) discovered by Stefanie, Jarolim et al. In the glucose medium, only the mother cell can divide to produce daughter cells, and the daughter cells cannot produce offspring, so just count the number of daughter cells under the microscope. The replicative lifespan of K6001 yeast cells could be determined. Therefore, K6001 yeast cells are very suitable as a model organism for screening anti-aging compounds. The present invention found that new ergo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com