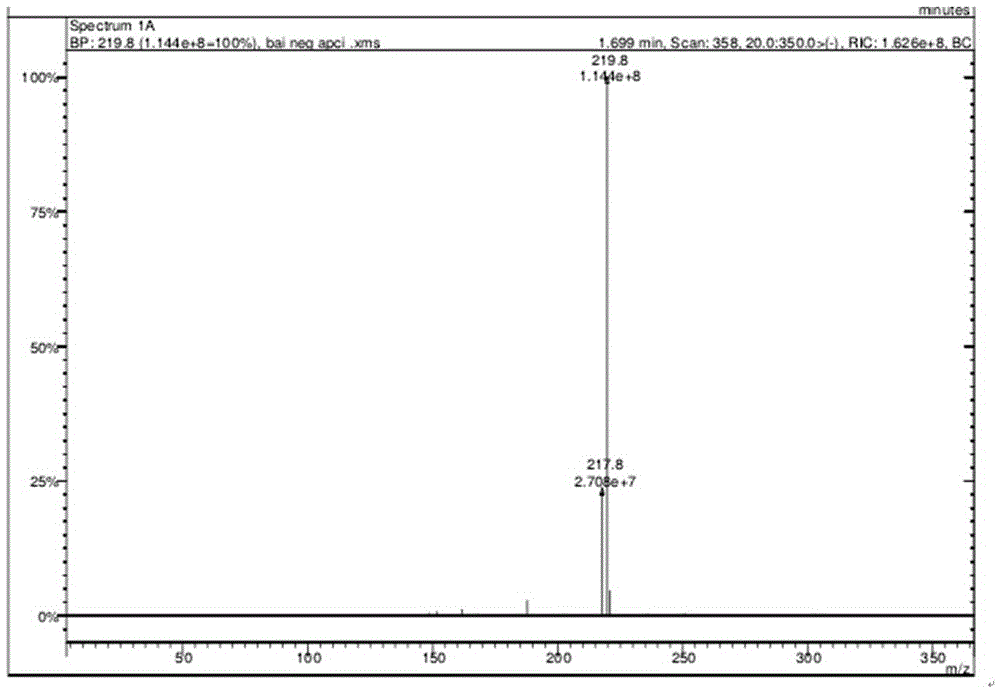
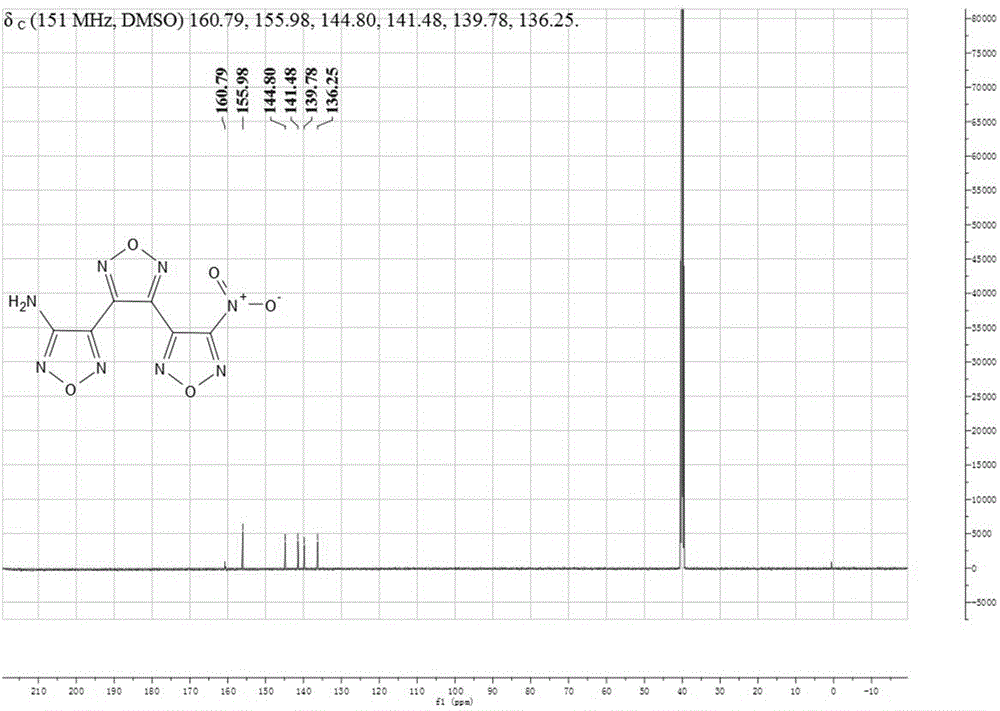
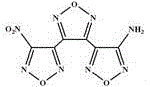
Synthetic method for 3-(4-aminofurazan-3-radical)-4-(4-nitrofurazan-3-radical) furazan
A technology of aminofurazan and nitrofurazan, applied in the direction of organic chemistry, can solve the problems of large thermal expansion and contraction, difficult melting and casting process, hidden safety hazards, etc., and achieve the goal of reducing usage, improving safety and yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Synthesis of intermediate 3,4-bis(3′-aminofurazan-4′-yl)furazan
[0028] At room temperature, dissolve 3,4-bis(3'-aminofurazan-4'-yl)furoxan (10g) in anhydrous methanol, stir to dissolve, then add concentrated hydrochloric acid (22ml), add in batches Stannous chloride dihydrate (27g) was reacted under reflux for 3h, cooled, a yellow solid precipitated, filtered off with suction, washed with ice water, and dried under vacuum to obtain 4.0g of a pale yellow solid BATF, with a yield of 42.7%.
[0029] (2) Synthesis of 3-(4-aminofurazan-3-yl)-4-(4-nitrofurazan-3-yl)furazan
[0030] At 0℃, dissolve sodium tungstate (2.94g) in 98% concentrated sulfuric acid (4.4ml), stir to dissolve, slowly add 30% H dropwise 2 O 2 (10.2ml), the temperature of the dripping process should not exceed 10℃, and stir. After the configuration is completed, heat up to 20°C, add 3,4-bis(3′-aminofurazan-4′-yl)furazan (2.36g), keep it for 3h, let stand for a period of time to filter, add the filtrate to ...
Embodiment 2
[0032] (1) Synthesis of intermediate 3,4-bis(3′-aminofurazan-4′-yl)furazan
[0033] At room temperature, dissolve 3,4-bis(3'-aminofurazan-4'-yl)furoxan (10g) in anhydrous methanol, stir to dissolve, then add concentrated hydrochloric acid (22ml), add in batches Stannous chloride dihydrate (27g) was reacted under reflux for 3.5h, cooled, and a yellow solid precipitated, filtered off with suction, washed with ice water, and dried in vacuo to obtain 5.8 g of BATF as a pale yellow solid, with a yield of 62.0%.
[0034] (2) Synthesis of 3-(4-aminofurazan-3-yl)-4-(4-nitrofurazan-3-yl)furazan
[0035] At 0℃, dissolve sodium tungstate (2.94g) in 98% concentrated sulfuric acid (8.1ml), stir to dissolve, slowly add 25% H dropwise 2 O 2 (18.7ml), the temperature of the dripping process does not exceed 10 ℃, and stir. After the configuration is completed, heat up to 25°C, add 3,4-bis(3'-aminofurazan-4'-yl)furazan (2.36g), keep it for 4h, let it stand for a period of time to filter, and add the ...
Embodiment 3
[0037] (1) Synthesis of intermediate 3,4-bis(3′-aminofurazan-4′-yl)furazan
[0038] At room temperature, dissolve 3,4-bis(3'-aminofurazan-4'-yl)furoxan (10g) in anhydrous methanol, stir to dissolve, then add concentrated hydrochloric acid (22ml), add in batches Stannous chloride dihydrate (30g) was reacted under reflux for 4.5h, cooled, a yellow solid precipitated, filtered off with suction, washed with ice water, and dried in vacuo to obtain 5.0g of a pale yellow solid BATF, with a yield of 53.4%.
[0039] (2) Synthesis of 3-(4-aminofurazan-3-yl)-4-(4-nitrofurazan-3-yl)furazan
[0040] At 0℃, dissolve sodium tungstate (2.94g) in 98% concentrated sulfuric acid (8.1ml), stir to dissolve, slowly add 25% H dropwise 2 O 2 (18.7ml), the temperature of the dripping process does not exceed 10 ℃, and stir. After the configuration is completed, heat up to 25°C, add 3,4-bis(3'-aminofurazan-4'-yl)furazan (2.36g), keep it for 4h, let it stand for a period of time to filter, and add the filtrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com