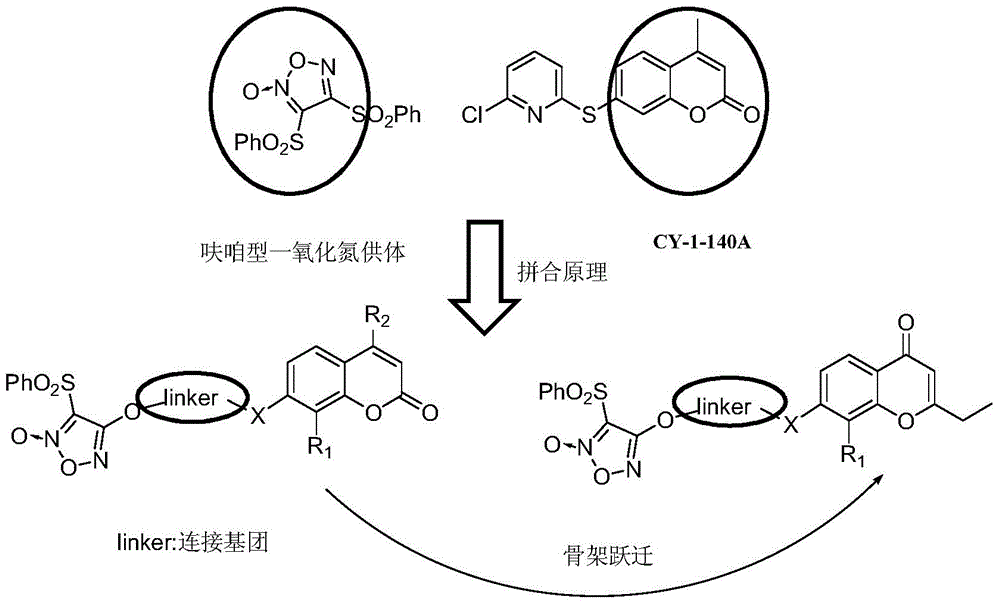
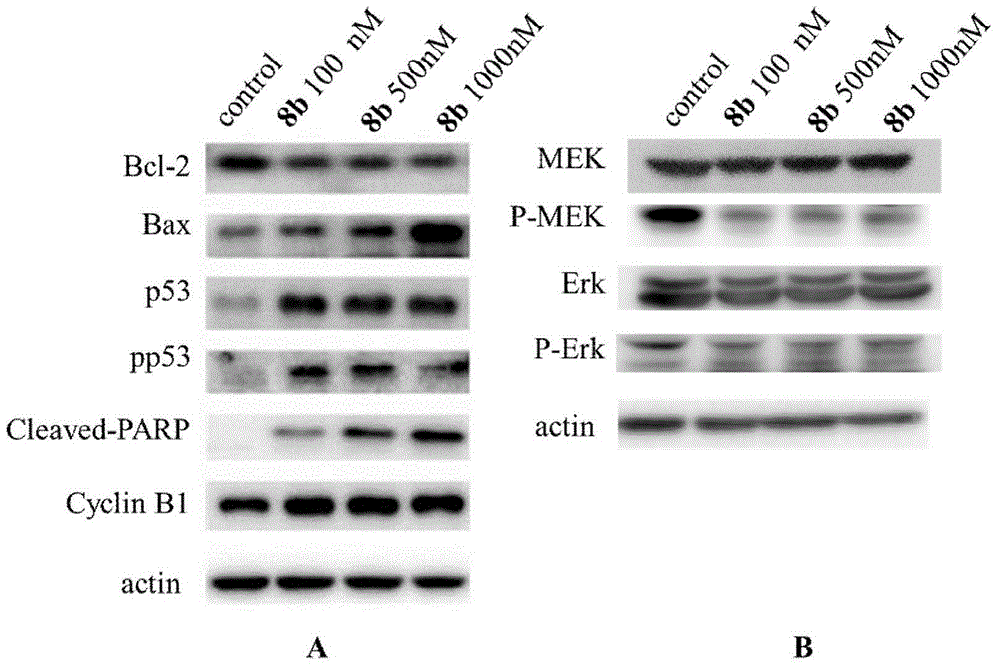
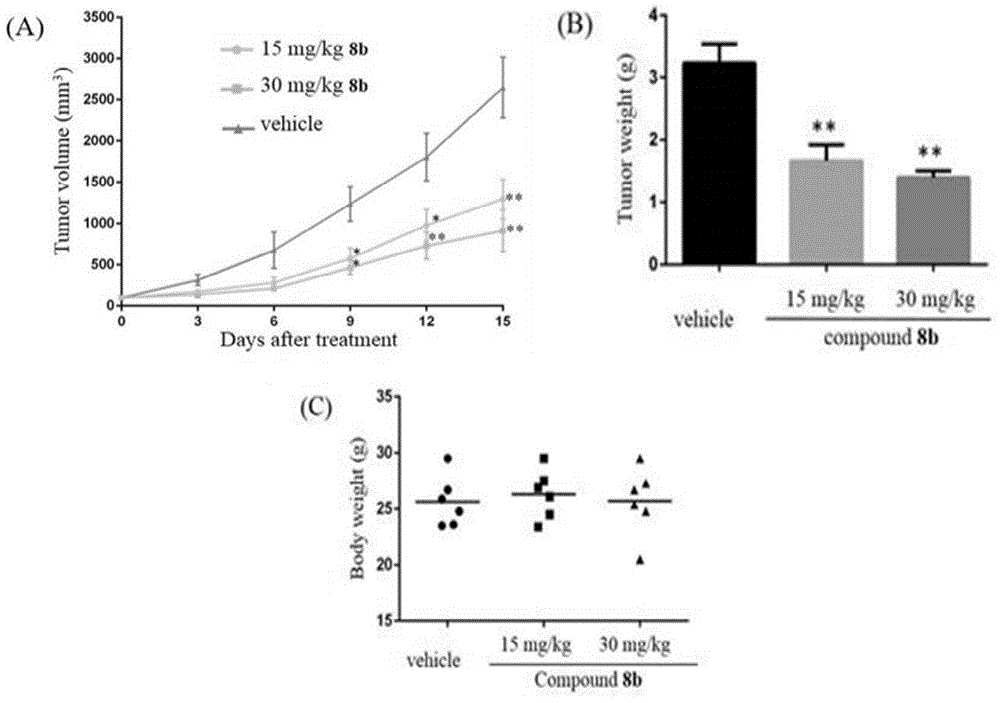
Furazan derivative of coumarin parent nucleus and antineoplastic activity
A technology of coumarin and derivatives, which is applied in the direction of antineoplastic drugs, organic active ingredients, drug combinations, etc., can solve the problems of drug resistance, reduced selectivity, and severe toxic and side effects, and achieve the activity inhibition of tumor cells, The effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A compound 4-(4-methyl-2H-chromene-2-dione-7-oxyl group)-3-(benzenesulfonyl)-1,2,5-oxa in compound 6a-c in general formula 2 Synthesis of oxadiazole 2-oxide (6b)
[0047] 7-Hydroxy-4-methyl-coumarin (5b, 300mg, 1.2mmol) and benzenesulfonylfurazan N-oxide (4, 256mg, 1.0mmol) and DBU (2.0mmol) in dichloromethane (6mL ) solvent, react at room temperature for 3 hours; then wash away the DBU with 3×5ml water, distill off the solvent under reduced pressure to obtain an oily liquid. A small amount of methanol was added to precipitate a white solid, which was filtered and recrystallized with ethyl acetate to obtain product 6b (320mg), with a yield of 90% and a melting point of 201-203°C. ESI-MSm / z 401.0[M+H] + ; 1 HNMR (400MHz, CDCl 3 )δ2.46(s,CH 3 C=),6.32(s,1H,-CH 3 C=CHCOO), 7.31(m, 2H, ArH), 7.68(m, 3H, ArH), 7.81(t, 1H, ArH, J=6.4Hz), 8.10(d, 2H, ArH, J=7.48Hz) .
Embodiment 2
[0049] Compound 4-(2-ethyl-4H-chromene-4-one-7-oxyl group)-3-(benzenesulfonyl)-1,2,5-oxadiazole 2-oxide ( 15) Synthesis of
[0050] 7-Hydroxy-2-ethyl-coumarin (14, 300mg, 1.2mmol) and benzenesulfonylfurazan N-oxide (4, 256mg, 1.0mmol) and DBU (2.0mmol) in dichloromethane (6mL ) solvent, react at room temperature for 3 hours; then wash away the DBU with 3×5ml water, distill off the solvent under reduced pressure to obtain an oily liquid. A small amount of methanol was added, and a white solid was precipitated. After filtration, it was recrystallized with ethyl acetate to obtain product 15 (280 mg). The yield is 89%, and the melting point is 159-161°C. ESI-MSm / z415.0[M+H] + ; 1 HNMR (400MHz, CDCl 3 )δ1.32(t,3H,CH 2 CH 3 , J=7.56Hz), 2.47(q,2H,CH 2 CH 3, J=7.56Hz), 6.21(s, 1H, OC=CH), 7.32(dd, 1H, ArH, J=8.80, 2.36Hz), 7.46(d, 1H, ArH, J=2.32Hz), 7.66( t,2H,ArH,J=8.28Hz),7.81(t,1H,ArH,J=7.56Hz),8.08(dd,2H,ArH,J=1.28,8.48Hz),8.27(d,1H,ArH, J = 8.80 Hz).
Embodiment 3
[0052] Synthesis of a compound 4-methyl-7-hydroxyethylamino-coumarin (7g) in compound type 7a-g in general formula two
[0053] Starting material 4-methyl-7-amino-coumarin (5e, 300mg, 1.56mmol), 2-bromoethanol (975mg, 7.80mmol), potassium carbonate (372mg, 4.68mmol) and potassium iodide (51mg, 0.31mmol) were dissolved In acetone (10 mL), reflux reaction for 10 hours, after filtration, the solvent was distilled off under reduced pressure, separated and purified by chromatography to obtain 7 g (212 mg) of a light yellow solid product, with a yield of 40%. ESI-MSm / z220.1[M+H] + ; 1 HNMR (400MHz, DMSO-d6) δ7.40 (d, 1H, ArH, J = 8.7Hz), 6.61 (dd, 1H, ArH, J = 8.7, 1.7Hz), 6.41 (d, 1H, ArH, J = 1.7Hz), 5.89(s, 1H, -CH=), 3.54(t, 2H, -OCH 2 CH 2 O-,J=5.8Hz),3.19–3.10(m,2H,-OCH 2 CH 2 O-),2.28(s,3H,-CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com