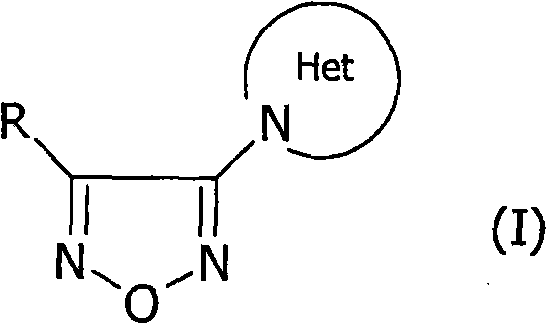
Furazane derivatives, preparation thereof and energetic compositions containing them
A composition and compound technology, applied in the direction of organic chemistry, etc., can solve the problems such as high-performance application of furoxan that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
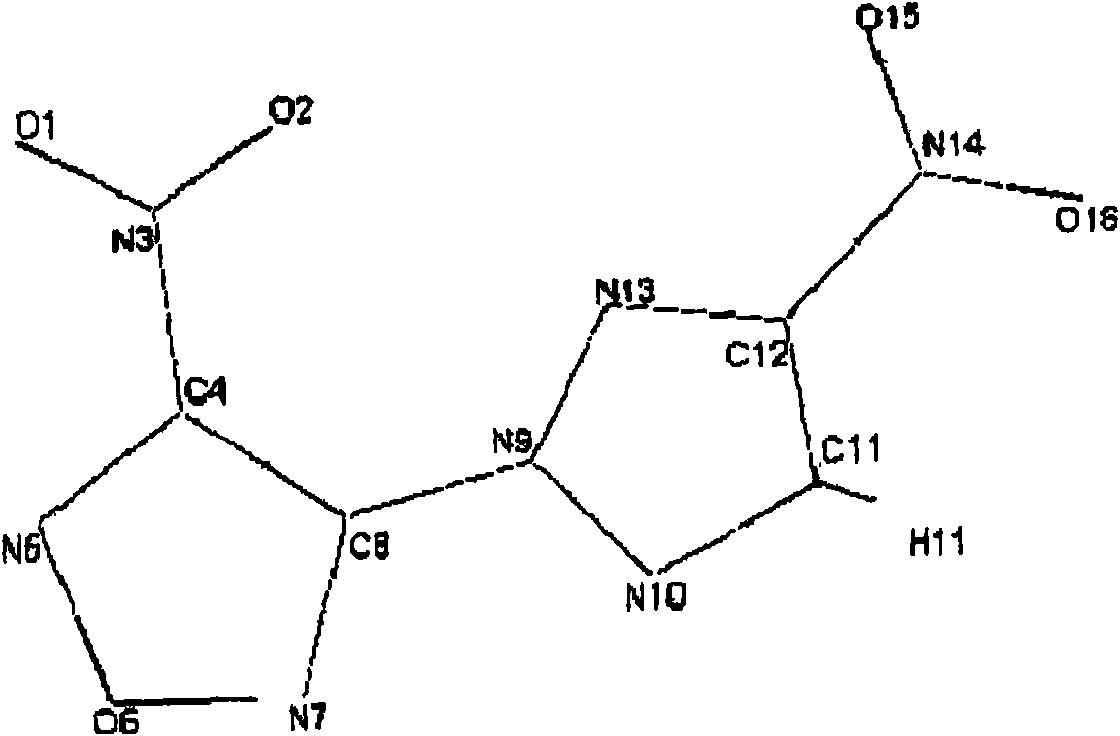
[0125] Place 5 ml of anhydrous acetonitrile in a dry three-necked flask under argon, then add 32.6 mg (1.358 mmol) of NaH, followed by 154 mg (1.35 mmol) of 3-nitro-1,2,4-triazole , the mixture was stirred at room temperature for 15 minutes to complete the formation of the nitrotriazolium anion. Dinitrofurazanyl ether (300 mg, 1.23 mmol) was added in one portion and the medium was stirred at room temperature for 20 hours. 20 ml of water are then added. The product was extracted with chloroform, the chloroform extracts were dried over magnesium sulfate, filtered and evaporated. The product was purified on silica gel. 194 mg of pure product were thus isolated (63% yield).
[0126] The expanded formula of the product and the physicochemical evidence for its structure ( 1 H. 13 C and 14 The results of the N NMR) analysis are shown in the first column of Table 1 below.
Embodiment 2
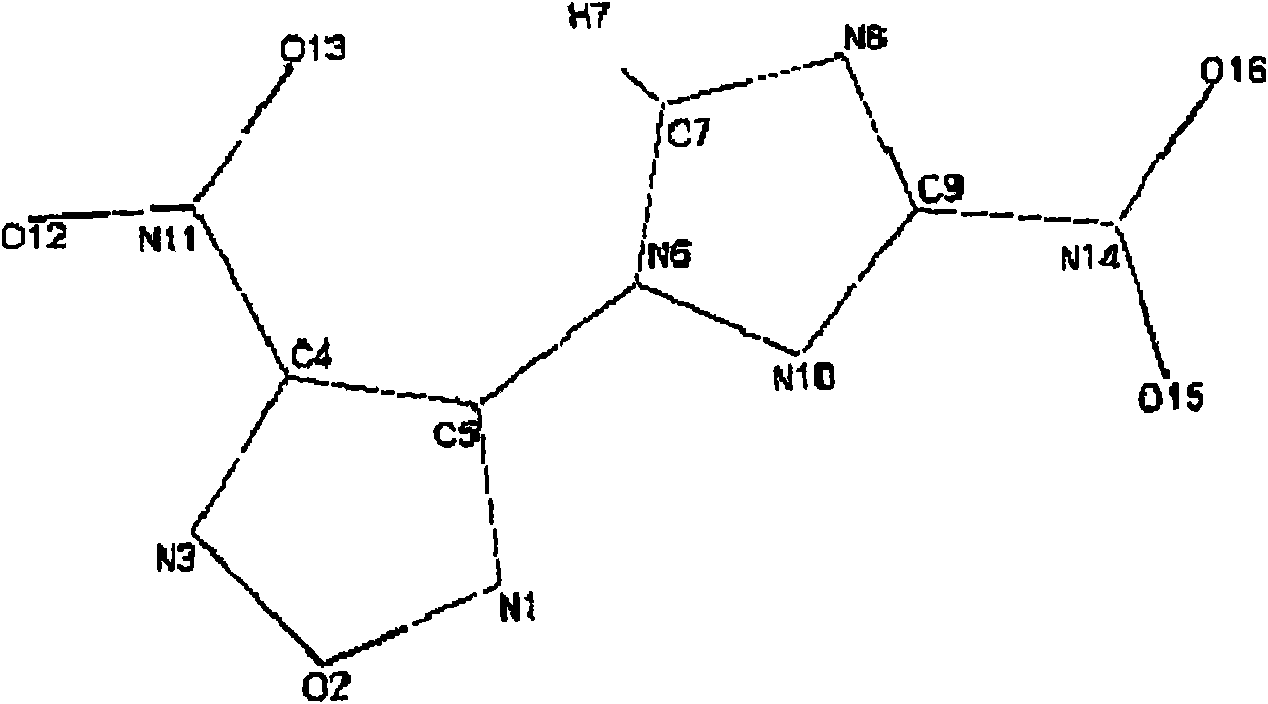
[0128] Put 5 ml of anhydrous acetonitrile into a dry three-necked flask under argon, then add 165.4 mg (1.03 mmol) of dinitrofurazan, followed by 117.1 mg (1.008 mmol) of 3-nitro-1,2, 4-triazole. 3 ml of a solution of pyridine (92 mg) diluted in acetonitrile are added dropwise to the reaction medium at 20°C. The medium is stirred at room temperature for 5 hours. 20 ml of water are then added. The product was extracted with chloroform, the chloroform extracts were dried over magnesium sulfate, filtered and evaporated. The product was purified on silica gel. 56 mg of pure product were thus isolated (24% yield relative to dinitrofurazan).
[0129] The product obtained is identical to that obtained in Example 1 (see first column of Table 1 below).
Embodiment 3
[0131] Add 2.05g of aminonitrofurazan (15mmol) to a solution of concentrated sulfuric acid (12ml) and concentrated phosphoric acid (12ml) at 0°C-5°C. Sodium nitrite (1.10 g, 15.8 mmol) was added in small portions. 1.64 g of nitroglycetaldoxime are added in one portion to the reaction medium. The reaction medium is stirred for 1 hour and then poured onto 40 g of ice. Separation by precipitation was allowed to occur for several hours, then the intermediate was filtered off, rinsed with water, and subsequently washed with P 2 o 5 dry. 0.46 g of the previously obtained dry product was put into 15 ml of distilled water. 0.75 ml of acetic anhydride was slowly flowed in at 20°C while maintaining the pH at 7 by adding 5% sodium hydroxide. At the end of the addition, the medium is acidified with 10% HCl solution. The product was extracted with ethyl acetate, the ethyl acetate extracts were dried over magnesium sulfate, filtered and evaporated. The product was purified on silica ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com