Gemcitabine/FTA/furazan conjugate in NO-donor type, preparation method and application
A technology of gemcitabine and conjugates, applied in the field of application in the preparation of antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
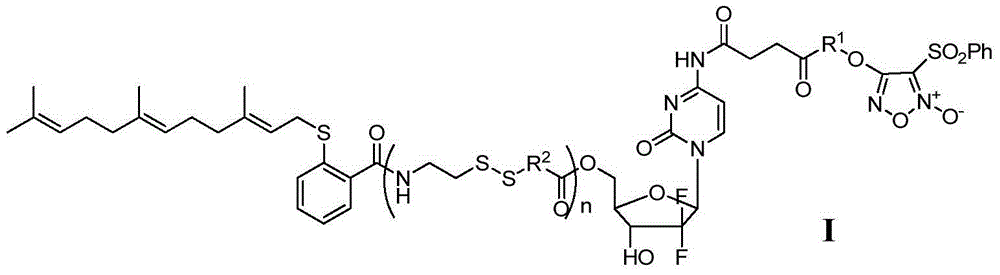
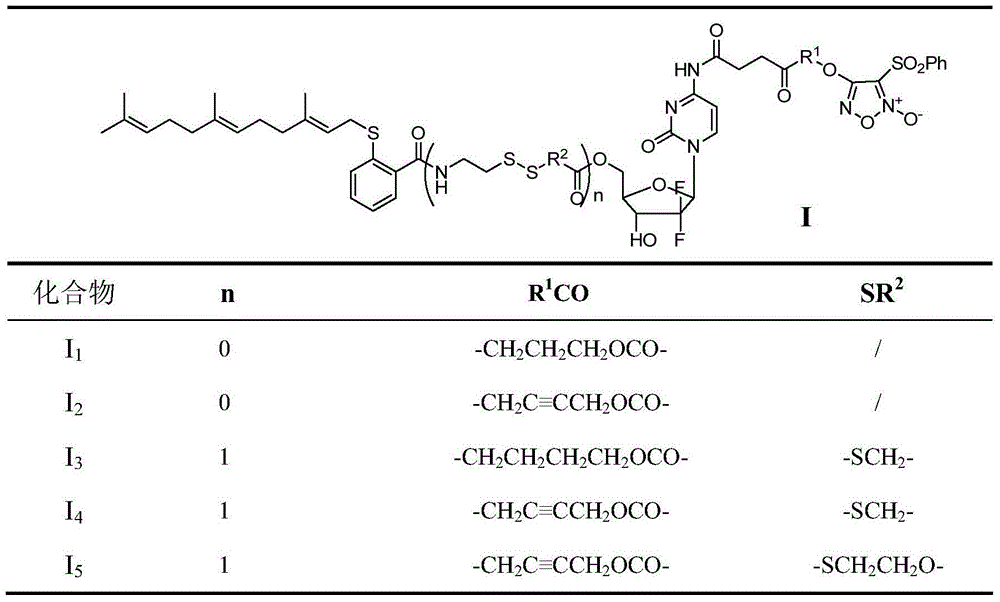
[0066] Example 1: 4-((4-((4-((1-((2R,4R,5R)-3,3-difluoro-4-hydroxyl-5-(((2-(((2E, 6E)-3,7,11-trimethyl-2,6,10-trien-1-yl)thio)benzoyl)oxy)methyl)tetrahydrofuran-2-yl)-2-oxo -1,2-dihydropyrimidin-4-yl)amino)-4-oxobutanoyl)oxy)propoxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole- 2-Oxide (I 1 ) preparation
[0067] 4-amino-1-((2R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy )methyl)-3,3-difluoro-tetrahydrofuran-2-yl)pyrimidin-2(1H)-one (3)
[0068] Gemcitabine 2 (2.63g, 10.00mmol) was dissolved in 200mL dry DMF solution, TBDMS-Cl (6.00g, 40.00mmol) and imidazole (2.72g, 40.00mmol) were added, stirred at room temperature for 18h, and the solvent was distilled off under reduced pressure. The crude product was purified by column chromatography (mobile phase methanol:ethyl acetate=1:20) to obtain 4.28 g of white solid with a yield of 87.3%. ESI-MS(m / z):491[M+H] + .
[0069] 4-(2-((4-((1-((2R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsily...
Embodiment 2
[0075] Example 2: 4-((4-((4-((1-((2R,4R,5R)-3,3-difluoro-4-hydroxyl-5-(((2-(((2E, 6E)-3,7,11-trimethyl-2,6,10-trien-1-yl)thio)benzoyl)oxy)methyl)tetrahydrofuran-2-yl)-2-oxo -1,2-Dihydropyrimidin-4-yl)amino)-4-oxobutanoyl)oxy)but-2-yn-1-yl)oxy)-3-(phenylsulfonyl)-1 , 2,5-oxadiazole-2-oxide (I 2 ) preparation
[0076] 4-((4-((4-((1-((2R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldi Methylsilyl)oxy)methyl)-3,3-difluorotetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)amino)-4-oxo Butyryl)oxy)but-2-yn-1-yl)oxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole-2-oxide (4b)
[0077] Referring to the synthetic method of (4a), 4-((4-((3-carboxypropionyl)oxy)but-2-yn-1-yl)oxy)-3-(phenylsulfonyl)-1, 2,5-Oxadiazole-2-oxide (1b) was reacted with NHS, DMAP, EDCI and compound 3 instead of (1a) to finally obtain light yellow solid (4b) with a yield of 49.3%.
[0078] 4-((4-((4-((1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2- Oxo-1,2-dihydropyrimidin-...
Embodiment 3
[0083]Example 3: 4-((4-((4-((1-((2R,4R,5R)-3,3-fluoro-4-hydroxyl-5-((2-((2-(2- (((2E,6E)-3,7,11-trimethyl-2,6,10-trienebutan-1-yl)thio)benzamido)ethyl)dithio)acetoxy Base)methyl)tetrahydrofuran-pyridin-2-yl)-2-oxo-1,2-dihydro-4-yl)amino)-4-oxobutanoyl)oxy)butoxy)-3- (Phenylsulfonyl)-1,2,5-oxadiazole-2-oxide (I 3 ) preparation
[0084] 4-((4-((4-((1-((2R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldi Methylsilyl)oxy)methyl)-3,3-difluorotetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)amino)-4-oxo Butyryl)oxy)but-2-yl)oxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole-2-oxide (4c)
[0085] Referring to the synthesis method of (4a), 4-(2-((3-carboxypropyl)oxy)butoxy)-3-(phenylsulfonyl)-1,2,5-oxadiazole-2- The oxide (1c) was reacted with NHS, DMAP, EDCI and compound 3 instead of (1a) to finally obtain a pale yellow solid (4c) with a yield of 51.0%.
[0086] 4-((4-((4-((1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2- Oxo-1,2-dihydropyrimi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com