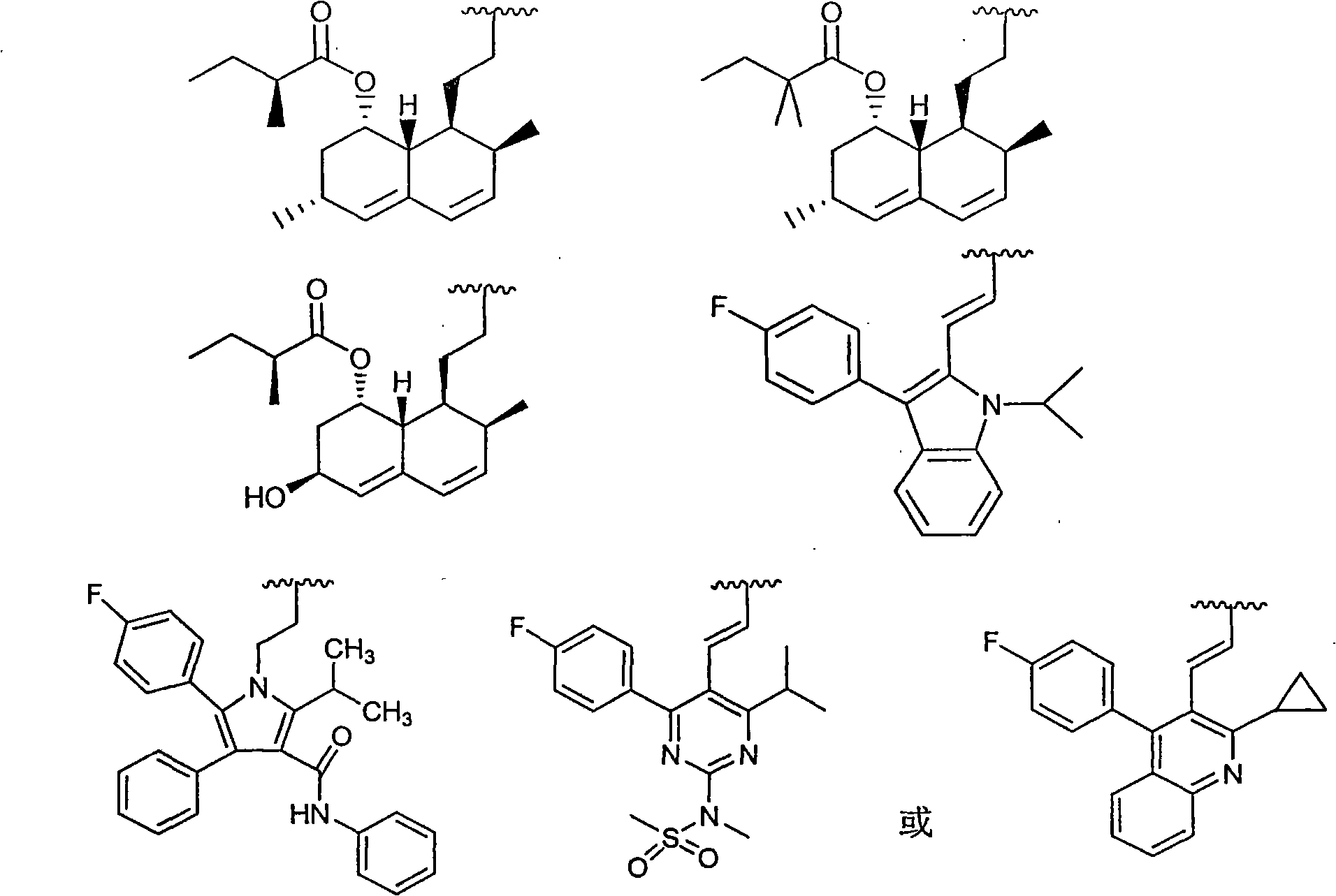
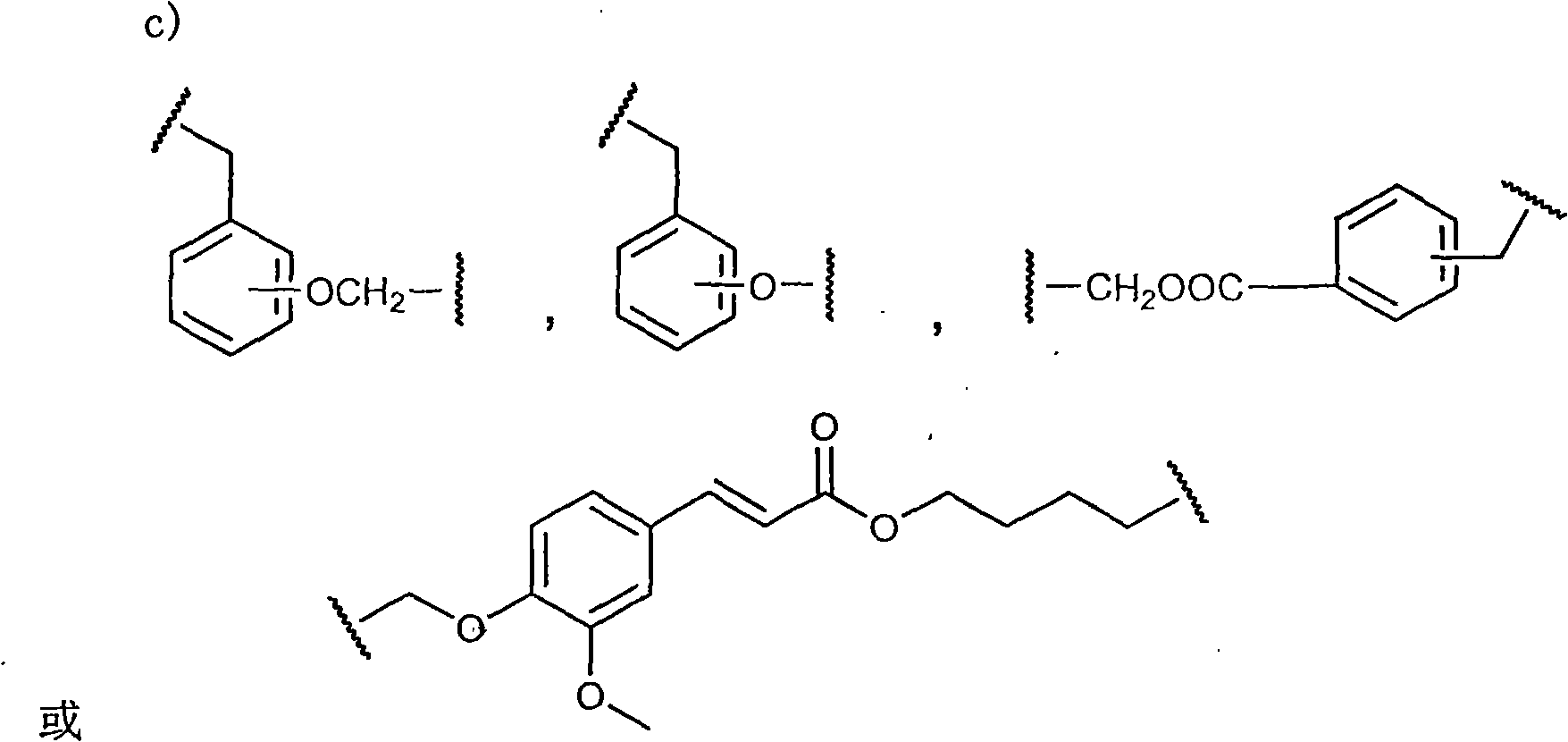
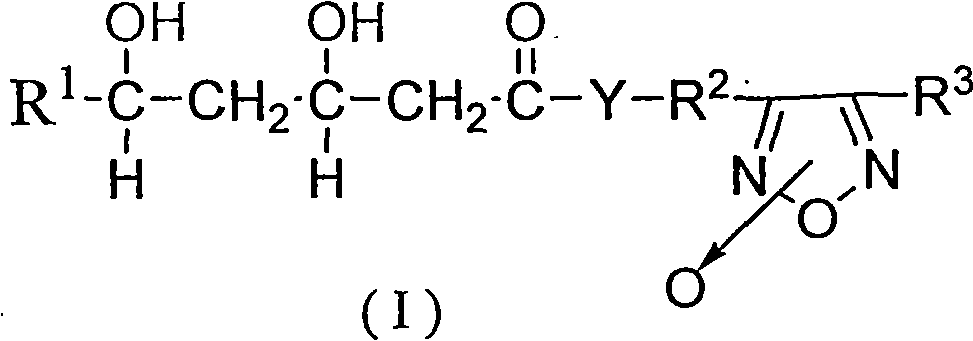Statins antilipemic drugs furazan nitroxides derivates and preparation method thereof
A technology of fluvastatin and pravastatin, which is applied in the field of furazanoxide derivatives of statin blood lipid-lowering drugs and its preparation, and can solve the problem of creatine kinase increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] (3R,5R)-3,5-dihydroxy-7-[(1S,2S,6R,8S,8αR)-2,6-dimethyl-8-(2,2-dimethylbutyryloxy )-1,2,6,7,8,8α-hexahydro-1-naphthyl]heptanoic acid-(4-phenyl-1,2,5-oxadiazole-2-oxide-3-)methanol Synthesis of Esters (Simvastatin (4-Phenyl-1,2,5-oxadiazole-2-oxide-3-) Methyl Ester)
[0103]
[0104] a) (3R, 5R)-3,5-dihydroxy-7-[(1S, 2S, 6R, 8S, 8αR)-2,6-dimethyl-8-(2,2-dimethylbutyryl Oxy)-1,2,6,7,8,8α-Hexahydro-1-naphthyl]heptanoate sodium (simvastatin sodium)
[0105] Add 1N sodium hydroxide solution (12ml) in the absolute ethanol (12ml) solution of simvastatin (5.0g, 12mmol), the reaction mixture was stirred and reacted at room temperature for 4 hours, and the reaction solution was decolorized by adding an appropriate amount of active carbon, and filtered The filtrate was concentrated under reduced pressure to obtain 4.7 g of a yellow-white solid.
[0106] b) (3R,5R)-3,5-dihydroxy-7-[(1S,2S,6R,8S,8αR)-2,6-dimethyl-8-(2,2-dimethylbutyryl Oxy)-1,2,6,7,8,8α-hexahydro-1-naphthyl]h...
Embodiment 2
[0111] (3R,5R)-3,5-dihydroxy-7-[(1S,2S,6R,8S,8αR)-2,6-dimethyl-8-(2,2-dimethylbutyryloxy )-1,2,6,7,8,8α-hexahydro-1-naphthyl]heptanoic acid-4-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4 Synthesis of -)oxybutyl ester (simvastatin-4-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-)oxybutyl ester)
[0112]
[0113] a) (3R, 5R)-3,5-dihydroxy-7-[(1S, 2S, 6R, 8S, 8αR)-2,6-dimethyl-8-(2,2-dimethylbutyryl Oxy)-1,2,6,7,8,8α-Hexahydro-1-naphthyl]heptanoic acid sodium
[0114] The preparation method is the same as in Example 1a).
[0115] b) (3R,5R)-3,5-dihydroxy-7-[(1S,2S,6R,8S,8αR)-2,6-dimethyl-8-(2,2-dimethylbutyryl Oxy)-1,2,6,7,8,8α-hexahydro-1-naphthyl]heptanoic acid-4-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide -4-)Oxybutyl ester
[0116] N, N'-dimethylformamide ( 5.0ml) solution was added dropwise to a solution of simvastatin sodium (0.33g, 0.8mmol) in N,N'-dimethylformamide (5.0ml), and the reaction mixture was stirred at room temperature for 24h. The reaction solution ...
Embodiment 3
[0120] (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-5-isopropyl-4-(phenylcarbamoyl)pyrrole-1-]-3,5-dihydroxy Heptanoic acid-(4-phenyl-1,2,5-oxadiazole-2-oxide-3-)methyl ester (atorvastatin-(4-phenyl-1,2,5-oxadiazole -Synthesis of 2-oxide-3-)methyl ester)
[0121]
[0122] A solution of 2-oxo-3-chloromethyl-4-phenyl-1,2,5-oxadiazole (0.38g, 1.8mmol) in N,N'-dimethylformamide (5.0ml) was gradually Add atorvastatin calcium (0.50 g, 1.2 mmol) in N, N'-dimethylformamide (5.0 ml) dropwise, and stir the reaction mixture at room temperature for 16 h. The reaction solution was treated with water and diethyl ether. The diethyl ether layer was dried over anhydrous magnesium sulfate and concentrated. The concentrate was purified by silica gel column (eluent: n-hexane / ethyl acetate 2 / 1) to obtain 0.68 g of the title compound as a yellow oil.
[0123] MS: 733.6(M+1) +
[0124] 1 H-NMR (CDCl 3 , 400MHz): 7.67-7.69(m, 2H), 7.52-7.58(m, 3H), 6.86-7.26(m, 14H), 5.14-5.22(m, 2H), 4.06-4.17(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com