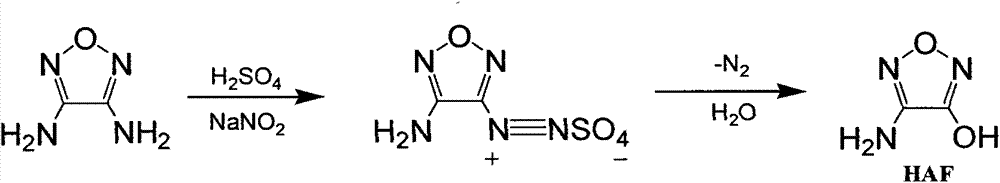
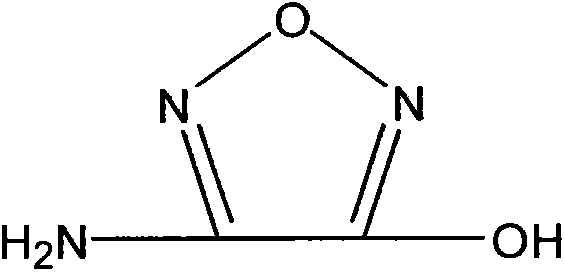
Synthetic method of 3-amino-4-hydroxyfurazan
A synthetic method and technology of hydroxyfuran, which is applied in the field of synthesis of 3-amino-4-hydroxyfuran, can solve the problems of low reaction yield, reduced reaction yield, increased by-products, etc., and achieve simple post-treatment process and high purity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 4g of 3,4-diaminofurazan and 45g of 24% (mass) sulfuric acid were added to the reaction flask under stirring, and at a temperature of -3°C to 3°C, 30mL of an aqueous solution containing 1.38g of sodium nitrite was added dropwise to the reaction flask. Control the dropping time for 2 hours. After adding the aqueous solution of sodium nitrite dropwise, react at a temperature of 0°C for 2 to 3 hours. At this time, the reaction liquid is pink; stop cooling and naturally heat up to 20°C to 25°C under stirring. Use a water bath to heat up to 50°C, and a large amount of nitrogen bubbles will emerge. After continuing to stir and react for 15 minutes, cool down in an ice-water bath to cool down to 0°C-5°C, stir for 30 minutes, and filter. The obtained filter cake is washed and vacuum-dried to After constant weight, 3.69 g of pale yellow-green solid 3-amino-4-hydroxyfurazan was obtained, with a yield of 91.4%.
[0021] Structure Identification:
[0022] Infrared (KBr, cm -1 ): ...
Embodiment 2
[0032] Add 4g of 3,4-diaminofurazan and 45g of 20% (mass) sulfuric acid into the reaction flask under stirring, and add 30mL of an aqueous solution containing 1.38g of sodium nitrite dropwise into the reaction flask at a temperature of -3°C to 3°C. Control the dropping time for 1.5h. After adding the aqueous solution of sodium nitrite dropwise, react at 0°C for 2-3h. At this time, the reaction liquid is pink; stop cooling and naturally heat up to 20°C-25°C under stirring. , use a water bath to heat up to 40°C, a large amount of nitrogen bubbles will emerge, continue to stir and react for 10 minutes, then cool down in an ice-water bath to cool down to 0°C-5°C, stir for 30 minutes, filter, and the obtained filter cake is washed and vacuum-dried After reaching a constant weight, 3.22 g of light yellow-green solid 3-amino-4-hydroxyfurazan was obtained, with a yield of 79.6%.
Embodiment 3
[0034] Add 4g of 3,4-diaminofurazan and 45g of 30% (mass) sulfuric acid into the reaction flask under stirring, and add 30mL of an aqueous solution containing 1.38g of sodium nitrite dropwise into the reaction flask at a temperature of -3°C to 3°C. Control the dropping time to 21.5 hours. After adding the aqueous solution of sodium nitrite dropwise, react at a temperature of 0°C for 2-3 hours. At this time, the reaction liquid is pink; stop cooling and naturally heat up to 20°C-25°C under stirring. , use a water bath to heat up to 40°C, a large amount of nitrogen bubbles will emerge, continue to stir and react for 30 minutes, then cool down in an ice-water bath to cool down to 0°C-5°C, stir for 30 minutes, filter, and the obtained filter cake is washed and vacuum-dried After reaching a constant weight, 3.38 g of light yellow-green solid 3-amino-4-hydroxyfurazan was obtained, with a yield of 83.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com