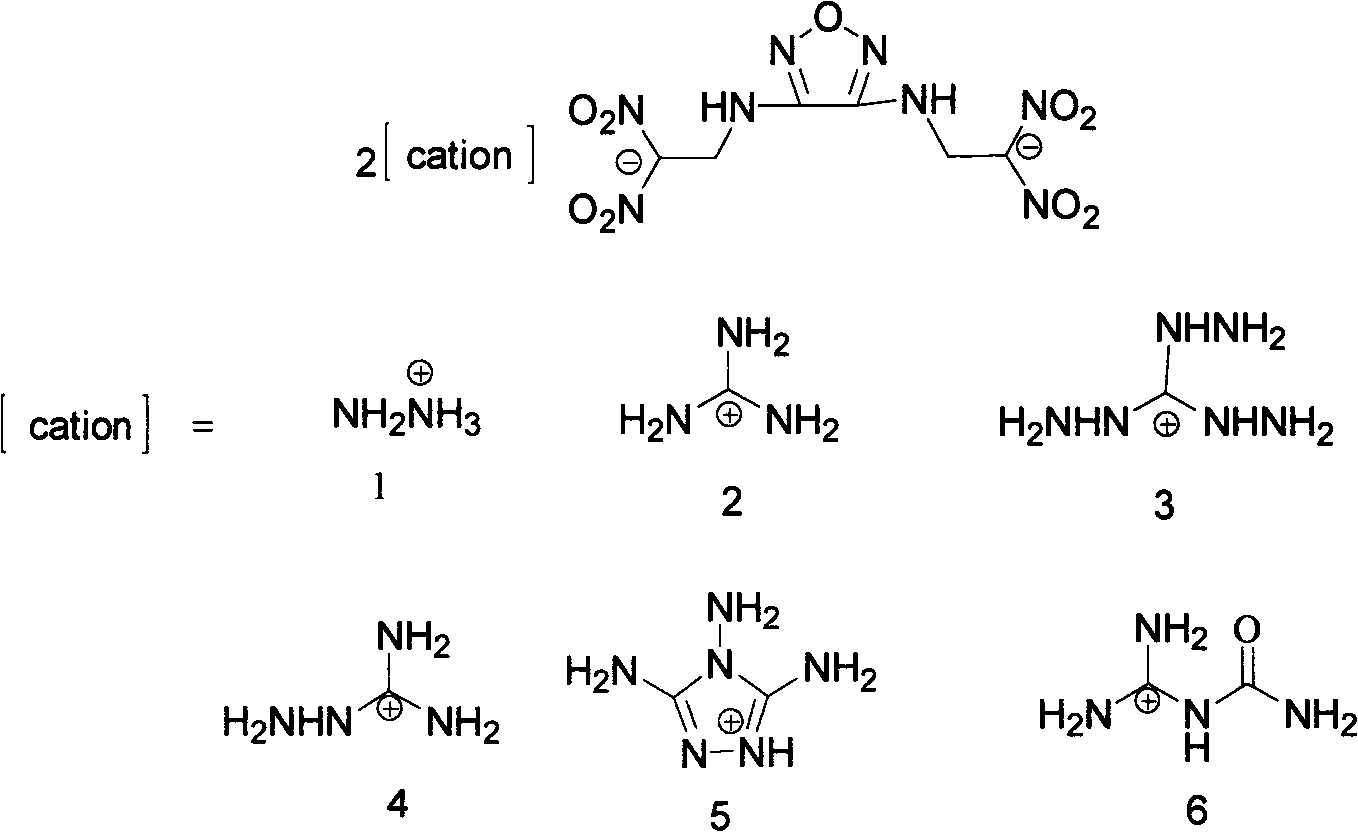
Diazo(N-dinitryl ethyl) furazan energy-containing ionic salts and preparation method thereof
A technology of dinitroethyl and dinitroethanol, which is applied in the field of heavy furazan energetic ion salts and its preparation, can solve the problems of unsatisfactory thermal stability and high sensitivity, achieve high yield, mild conditions, Ease of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
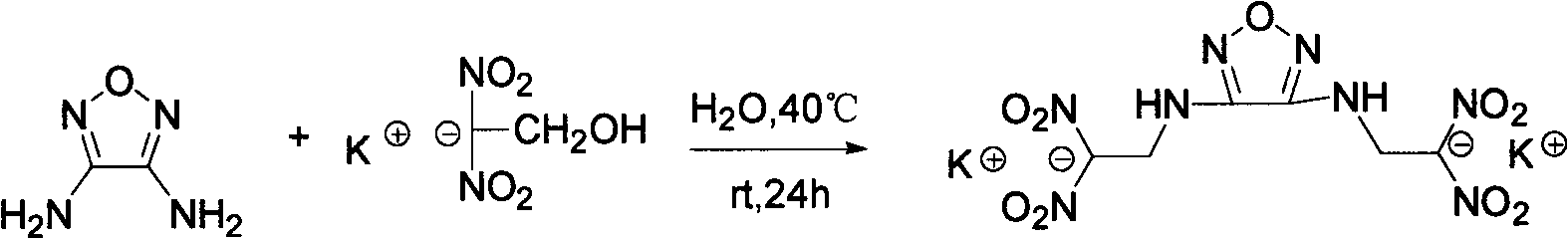
[0026] The preparation of embodiment 1 heavy (N-dinitroethyl) furazan potassium salt (1)
[0027] Weigh 1.74g (10mmol) of potassium dinitroethoxide, stir at room temperature for about 5min, and most of it is still undissolved, weigh 510mg (5.1mmol) of diaminofurazan and add it to the reaction solution, heat up to 40°C to react, about Half an hour later, almost all the solids were dissolved, and the solution turned into a yellow clear solution. The reaction was continued for 24 hours, filtered, and the solid product was washed with methanol. 1.96 g of solid was obtained with a yield of 95%.
[0028] Its structural formula is as follows:
[0029]
[0030] Decomposition temperature: 166°C (DSC). 1 H NMR (400MHz, d 6 -DMSO): δ=4.577(d, 2H), δ=6.191(t, 1H) ppm; 13 C NMR (100MHz, d 6 -DMSO): δ=152.13, 134.94, 45.14ppm; IR (KBr pellet): 3324, 3189, 1591, 1458, 1338, 1289, 1191, 1113, 751, 666cm -1 ;elemental analysis(%) calcd for C 6 h 6 K 2 N 8 o 9 (411.95): C 17.48, H...
Embodiment 2
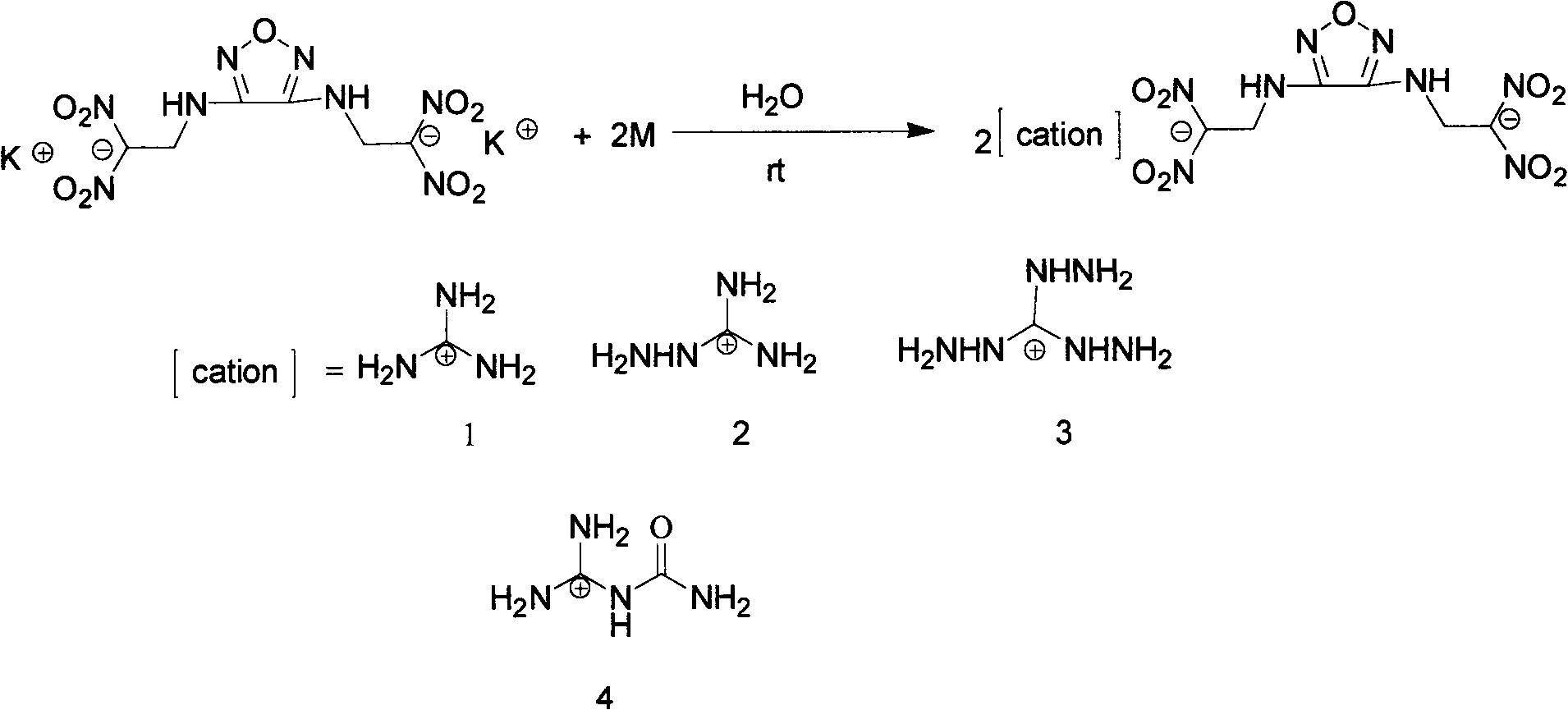
[0031] The preparation of embodiment 2 heavy (N-dinitroethyl) furazanguanidine salts (2)
[0032] Add 412 mg (1.0 mmol) heavy (N-dinitroethyl) furazan potassium salt and 5 mL H to a 25 mL single-necked flask 2 O was dissolved into a light yellow clear liquid, and then, with stirring at room temperature, was added 2 mL of H 2 Guanidine hydrochloride 190 mg (2.0 mmol) in O, reacted with stirring at room temperature, precipitated a light yellow solid precipitate, the reaction mixture continued to stir at room temperature for 1 h, filtered the light yellow precipitate and washed twice with ice water and methanol, and the solid was placed in a desiccator at room temperature After drying for 2 h, 438 mg of the product was obtained with a yield of 96%.
[0033] The reaction conditions not mentioned in the following examples are the same as in Example 2.
[0034] Its structural formula is as follows:
[0035]
[0036] Decomposition temperature: 172°C (DSC). Density is 1.719g cm...
Embodiment 3
[0037] Embodiment 3 Heavy (N-dinitroethyl) Furazan 3,4,5-triamino-1,2,4-triazole salt preparation (3)
[0038] Add 412 mg (1.0 mmol) heavy (N-dinitroethyl) furazan potassium salt and 5 mL H to a 25 mL single-necked flask 2 O dissolves into light yellow clear liquid, add about 10ml CH 2 Cl 2 As a solvent, adjust the mixed solution to acidity with dilute hydrochloric acid to free neutral molecules from heavy (N-dinitroethyl)furazan potassium salt, separate the mixed solution, and wash the organic phase twice with water. Weigh 228 mg (2.0 mmol) of 3,4,5-triamino-1,2,4-triazole and dissolve it in methanol, and add the methanol solution dropwise to the above separated CH 2 Cl 2 phase, after stirring at room temperature for about 5 min, a light yellow solid precipitated immediately, continued stirring for 1 h, filtered the light yellow precipitate and washed twice with ice water and methanol, and dried the solid in a desiccator at room temperature for 2 h to obtain 500 mg of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com