Compound containing rhodamine groups and benzofurazan groups and preparation method and application thereof
A technology for benzofurazan and compounds, which is applied in the fields of preparation and application of compounds containing rhodamine groups and benzofurazan groups, and achieves the effects of high accuracy and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
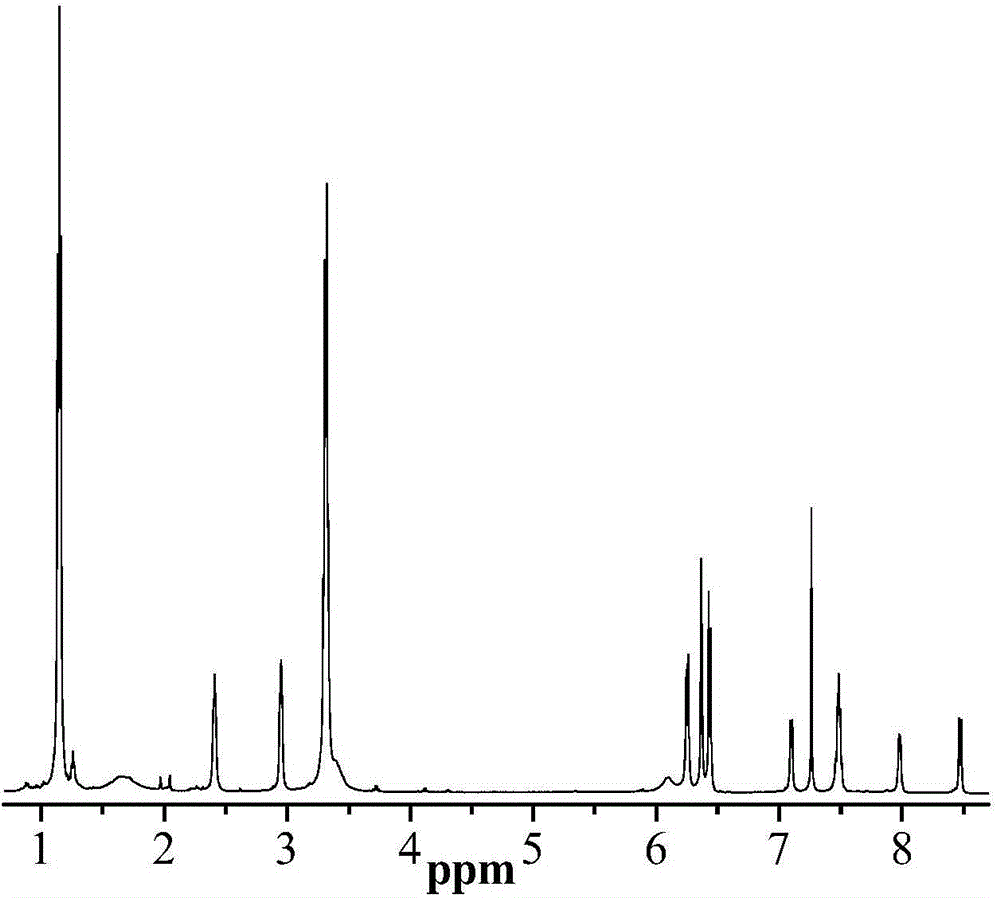
[0056] Synthesis of nitrogen-(7-nitrobenzofurazan)-rhodamine B-acyldiethylenetriamine
[0057]
[0058] (I)
[0059] The synthetic route of nitrogen-(7-nitrobenzofurazan)-rhodamine B acid diethylenetriamine is shown in formula (I), and the specific synthetic steps are as follows:
[0060] (1) Synthesis of rhodamine B-acyl diethylenetriamine
[0061] Dissolve Rhodamine B (0.5 g 1 mM) in a round bottom flask filled with 20 mL of ethanol, stir at room temperature for 5 minutes, after Rhodamine B is completely dissolved, slowly add 3 mL of diethylenetriamine dropwise, and reflux at 70°C for 12 hours , until the solution changed from red to orange-yellow. Remove the solvent, add dichloromethane to dissolve, and wash with distilled water until the water phase is colorless, collect, dry the organic layer and spin dry to obtain the product, which is directly used for the next reaction without further purification. Preliminary separation and purification
[0062] (2) Synthesis...
Embodiment 2
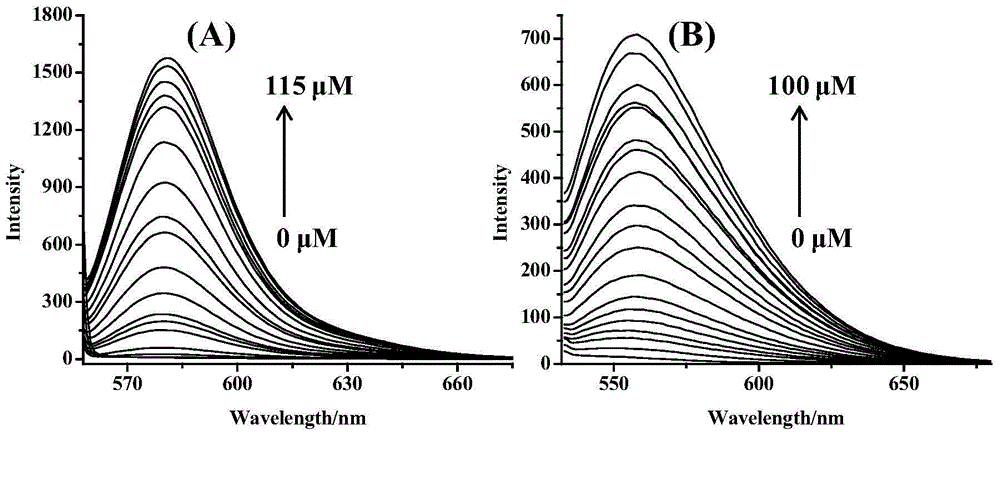
[0066] Titrate the concentrated solution of mercury ions to a concentration of 1.0 × 10 -5 In the ethanol solution of nitrogen-(7-nitrobenzofurazan)-rhodamine B-acyldiethylenetriamine of mol / L, the concentration of mercury ions is respectively 0.0, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 7.0, 10.0, 12.5.0, 15.0, 20.0, 25.0, 30.0, 40.0, 50.0, 60.0, 80.0, 100.0 μM (× 10 -6 mol / L), and respectively detect their fluorescence intensities at the excitation wavelength of 525 nm, draw the fluorescence spectrum diagram, the obtained fluorescence spectrum diagram is as follows image 3 In (A) shown.
[0067] Titrate the concentrated solution of iron ions to a concentration of 1.0 × 10 -5 mol / L of nitrogen-(7-nitrobenzofurazan)-rhodamine B-acyldiethylenetriamine contains 30% ethanol in the aqueous ethanol solution so that the concentration of iron ions is respectively 0.0, 5.0, 10.0, 15.0, 20.0, 25.0, 27.0, 30.0, 35.0, 40.0, 50.0, 60.0 , 70.0, 80.0, 90.0, 100.0, 115.0 μM (× 10 -6 mol / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com