A kind of iridium complex containing (hydrazinocarbonyl) ferrocene ligand and preparation method and application thereof
A technology of hydrazinocarbonyl and iridium complexes, applied in the field of fluorescent materials, can solve the problems of less research, achieve high sensitivity, reduce the proportion of side reactions, and shorten the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
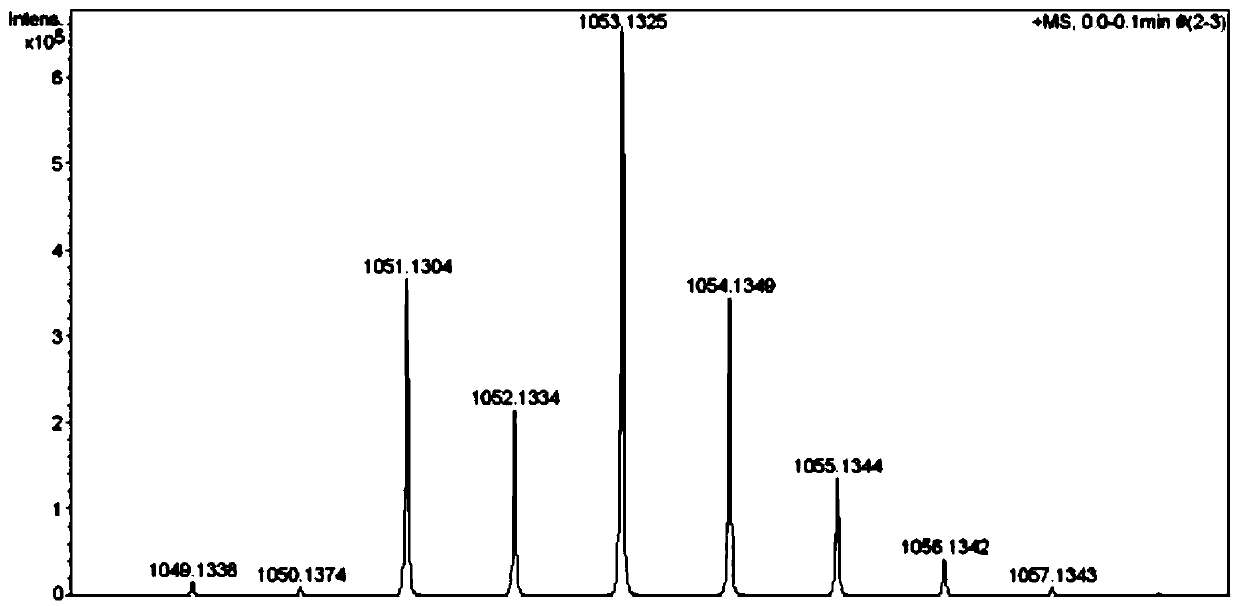
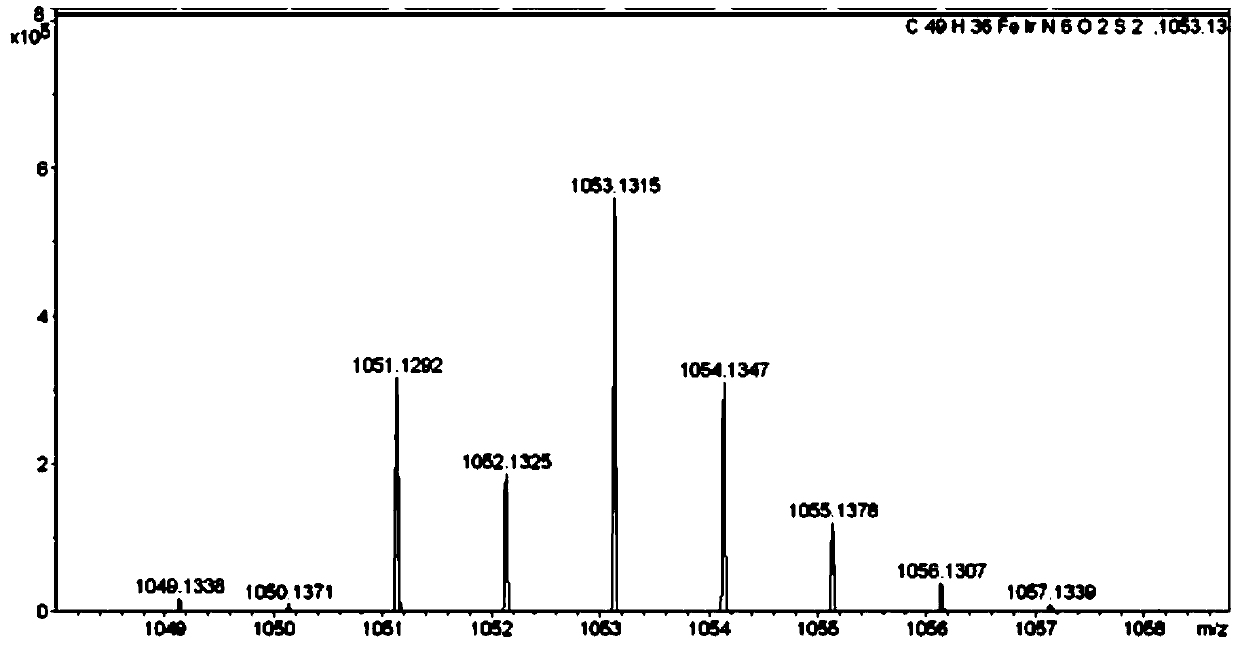
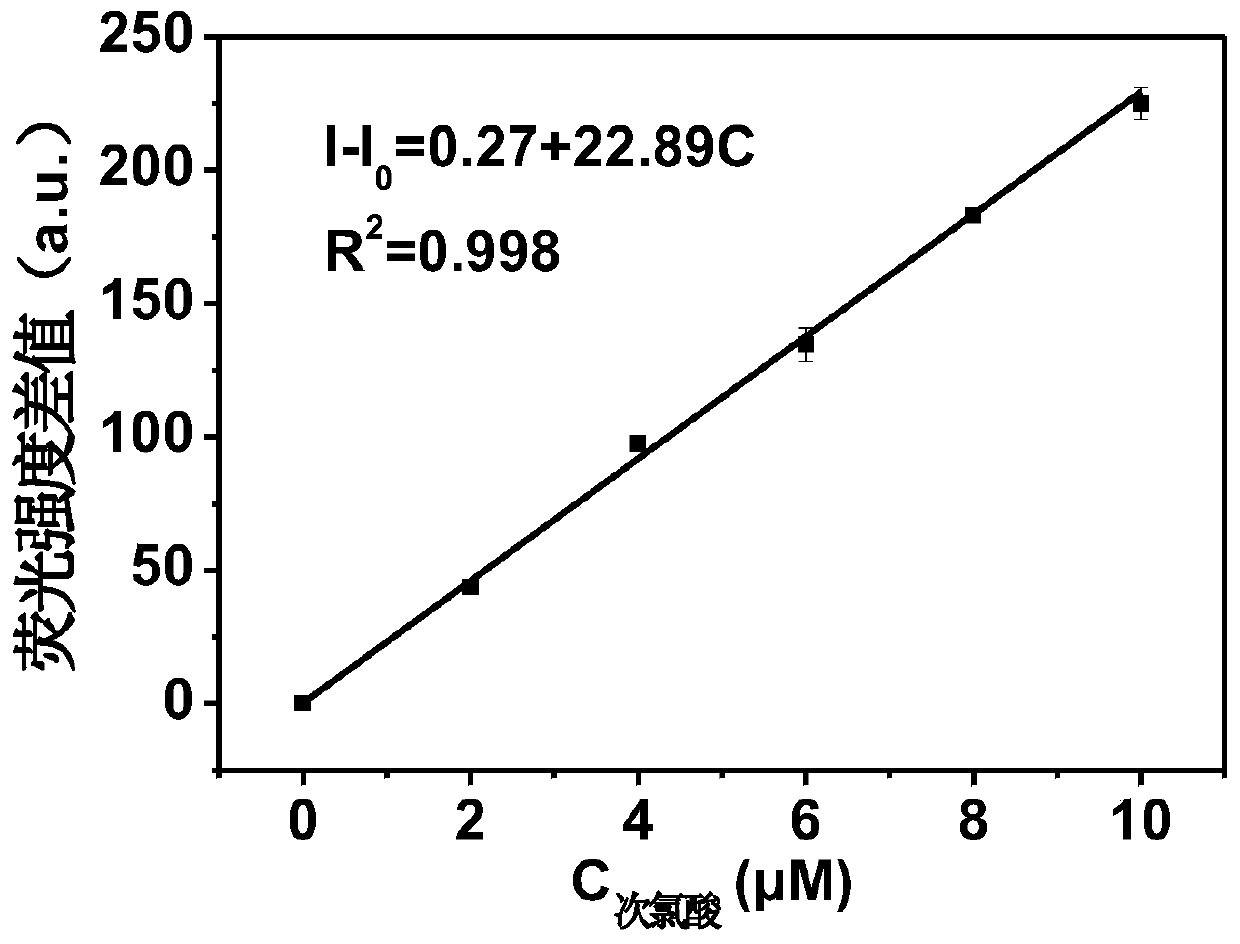
Image
Examples
Embodiment 1
[0038] The method for preparing the iridium complex containing (hydrazinocarbonyl) ferrocene ligand in this embodiment consists of the following steps:
[0039] (1) Preparation of chlorine-bridged dimers
[0040] Under nitrogen protection, 0.3528g (1mmol) of IrCl 3 ·3H 2 O was added to the mixed solvent of 2-ethoxyethyl ether and water, 2-ethoxyethanol: water (V:V) = 30:10mL, magnetically stirred for 15min, after stirring evenly, 0.4792g (2.2mmol) 2-Phenylbenzothiazole, heated to 120°C, refluxed, stirred for 24 hours, then stopped the reaction, cooled to room temperature, and filtered with suction to obtain a precipitate, which was washed with 30mL of secondary deionized water, 30mL of ethanol and 30mL of n-hexane , vacuum drying in a drying oven at 50°C for 12 hours to dryness to obtain an orange-red powdery chlorine-bridged dimer;
[0041] (2) Preparation of 4'-methyl-2,2'-bipyridine-4-(hydrazinocarbonyl)ferrocene
[0042] Under anhydrous and oxygen-free conditions, add 21...
Embodiment 2
[0048] The method for preparing the iridium complex containing (hydrazinocarbonyl) ferrocene ligand in this embodiment consists of the following steps:
[0049] (1) Preparation of chlorine-bridged dimers
[0050] Under nitrogen protection, 0.3528g (1mmol) of IrCl 3 ·3H 2 O was added to the mixed solvent of 2-ethoxyethyl ether and water, 2-ethoxyethanol: water (V:V) = 30:10mL, magnetically stirred for 15min, after stirring evenly, 0.4356g (2mmol) of 2-Phenylbenzothiazole, heated to 120°C, refluxed, stirred for 15 hours, then stopped the reaction, cooled to room temperature, and filtered with suction to obtain a precipitate, which was washed with 30mL secondary deionized water, 30mL ethanol and 30mL n-hexane respectively, Vacuum bake in a drying oven at 50°C for 12 hours to dryness to obtain an orange-red powdery chlorine-bridged dimer;
[0051] (2) Preparation of 4'-methyl-2,2'-bipyridine-4-(hydrazinocarbonyl)ferrocene
[0052] Under anhydrous and oxygen-free conditions, ad...
Embodiment 3
[0056] (1) Preparation of chlorine-bridged dimers
[0057] Under nitrogen protection, 0.3528g (1mmol) of IrCl 3 ·3H 2 O was added to the mixed solvent of 2-ethoxyethyl ether and water, 2-ethoxyethanol: water (V:V) = 30:10mL, magnetically stirred for 15min, after stirring evenly, 0.5445g (2.5mmol) 2-Phenylbenzothiazole, heated to 120°C, refluxed, stirred for 18 hours, then stopped the reaction, cooled to room temperature, and filtered with suction to obtain a precipitate, which was washed with 30mL of secondary deionized water, 30mL of ethanol and 30mL of n-hexane , vacuum drying in a drying oven at 50°C for 12 hours to dryness to obtain an orange-red powdery chlorine-bridged dimer;
[0058] (2) Preparation of 4'-methyl-2,2'-bipyridine-4-(hydrazinocarbonyl)ferrocene
[0059] Under anhydrous and oxygen-free conditions, add 214mg (1mmol) of 4'-methyl-2,2'-bipyridine-4-carboxylic acid into 50mL of thionyl chloride solvent, heat to reflux at 75°C, and stop after stirring for 3h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com