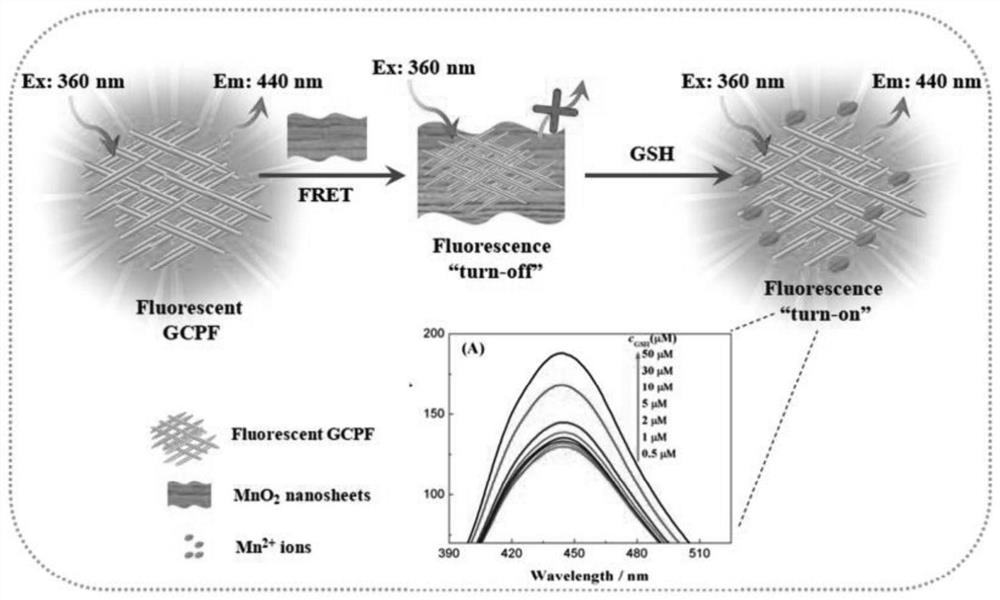
A method for detecting gsh using glutaraldehyde-chitosan non-conjugated fluorescent polymer as fluorescent probe
A fluorescent polymer and fluorescent probe technology, applied in the field of fluorescent detection, can solve the problems of high interference, low selectivity and low sensitivity of fluorescent probes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Fluorescent GCPF, MnO 2 Preparation of nanosheets and fluorescence detection of GSH
[0049] 1. Preparation of fluorescent GCPF
[0050] First, at room temperature, under vigorous stirring, dissolve 1.00g of chitosan in 100.0mL of water; secondly, add 1mL of 1.74M glacial acetic acid (HAc) solution drop by drop and stir continuously for 10 minutes; then quickly add 0.10mL of 4.73M glutaraldehyde; after continuous stirring for 6h, a yellow transparent fluorescent GCPF solution was obtained.
[0051] 2. Preparation of MnO2 nanosheets
[0052] First, 0.3M MnCl of 10mL will be placed in 15 seconds 2 ·4H 2 O Aqueous solution was added to 20 mL of 0.6 M tetramethyl ammonium hydroxide (TMA· OH) and 3wt%H 2 O 2 Then, after continuous and constant agitation at room temperature overnight, MnO is obtained by centrifugation 2 Nanosheets; will result in MnO 2 Nanosheet MnO 2 The nanosheets are washed three times with ultrapure water and methanol, centrifuged at 10,000 rpm for 10 min; fi...
Embodiment 2
[0059] GCPF and MnO 2 Structural characterization of nanosheets and their feasibility for fluorescence enhancement detection of GSH
[0060] The water-soluble GCPF yellow transparent solution is blue fluorescent, with a maximum excitation wavelength of 360 nm and a maximum emission wavelength of 440 nm. As can be seen by the determination of its fluorescence intensity, this GCPF solution can be stable for more than two months when stored in a refrigerator at 4 °C.
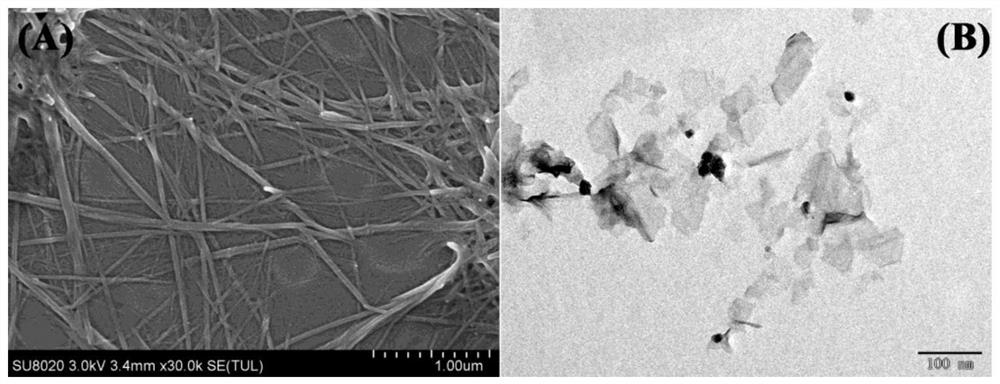
[0061] from Figure 2 As can be seen in the electron microscopy diagram shown: GCPF is shown as a nanorod or linear, with a vertical diameter of nearly 80 nm and a length of several microns ( Figure 1 A)。 MnO 2 Nanosheets appear as layered structures with two-dimensional characteristic morphology ( Figure 1 B)。
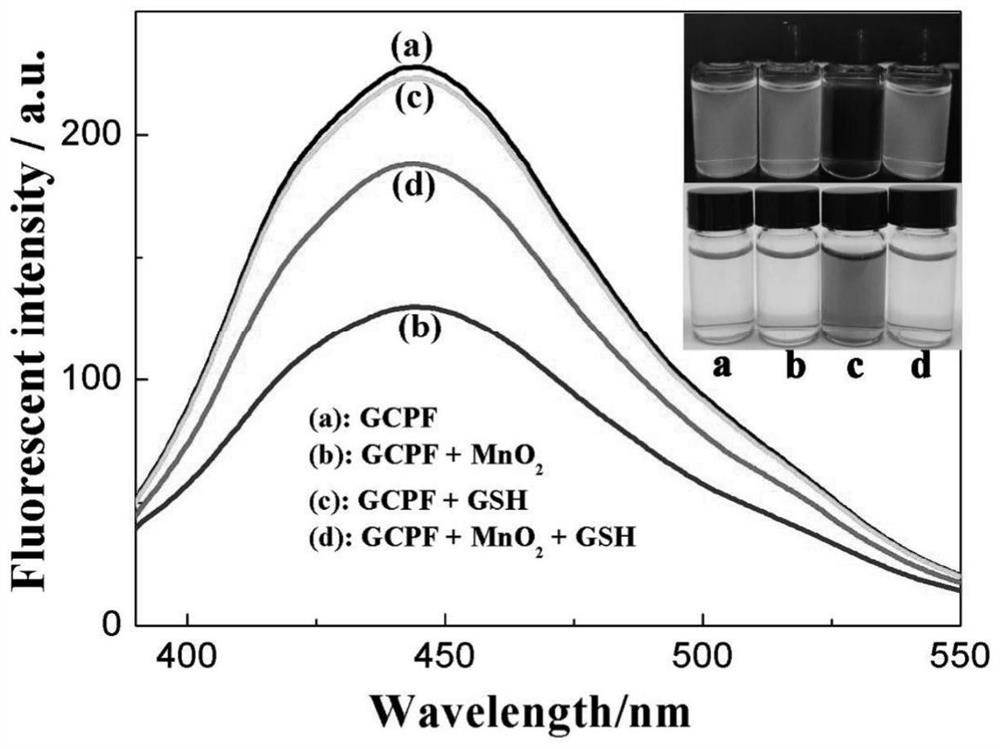
[0062] In order to confirm GCPF and MnO 2 Nanosheets are used for the feasibility of fluorescence enhancement to determine GSH, and we first did a preliminary experiment with 50mMGSH. The test conditions are:...
Embodiment 3
[0065] Condition optimization for fluorescence enhancement for the detection of GSH
[0066] The present embodiment optimizes different amounts of MnO 2 Effect of nanosheets on GSH detection. The results are as follows Figure 4 As shown, discovery is with MnO 2 The concentration of nanosheets increased from 0.005 to 0.5 mg / mL, and the fluorescence intensity of GCPF became weaker and weaker; after the addition of GSH, the fluorescence of GCPF gradually increased. For maximum signal-to-noise ratio, choose MnO of 0.05 mg / mL 2 Nanosheets are the best conditions for detecting GSH by fluorescence enhancement.
[0067] At the same time, the present embodiment optimizes the effect of different pH and temperature on the detection of GSH by fluorescence enhancement method. The results are as follows Figure 5 and Figure 6 as shown.
[0068] Considering the differences between fluorescence enhancement and quenching, the kinetic behavior of fluorescence enhancement method for glasmination enha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| degree of deacetylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com