Deoxyribonucleic acid (DNA) base analogue with photo-crosslinking group biaziridine and synthetic method thereof
A technology of photocrosslinking group and diaziridine, which is applied in the field of DNA base analogs and their synthesis, can solve the sites where the secondary structure is unknown and the interaction between nucleic acid aptamers and proteins is the strongest. and other problems, to achieve the effect of simple and feasible steps and cheap synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of intermediate product 2, its synthetic route is as follows:
[0032]
[0033] Add raw material 1 (105.3mg, 0.82mmol), 1-aminohexanediol (132mg, 0.902mmol), DCC (203mg, 0.984mmol), HOBt (133mg, 0.984mmol), 10mL solvent in a round bottom flask, in Under the protection of nitrogen, the reaction was carried out at room temperature for 24 hours. After the completion, it was separated and purified with a silica column, and characterized by NMR and mass spectrometry. 1 H NMR (500MHz, CDCl 3 )δ3.73(m,2H),3.61(m,2H),3.19(m,2H),1.98(t,2H),1.72(t,2H),1.66(m,1H),1.45(m,2H ),1.30-1.20(m,4H),0.99(s,3H).ESI-MS Calculated for C 12 h 23 N 3 o 3 Na:280.33([M+Na] + ), Found: 280.2.
Embodiment 2
[0035] The synthesis of intermediate product 3, its synthetic route is as follows:
[0036]
[0037] In a round bottom flask was added 2 (424.4mg, 1.65mmol), 4-dimethylaminopyridine (20mg, 0.165mmol), 10mL pyridine, under nitrogen protection. At the same time, under nitrogen protection, 4-4'-dimethoxytriphenylchloromethane (560mg, 1.65mmol) was dissolved in 4mL of dichloromethane, and the solution was added dropwise to the above solution under ice bath, and then After 24 hours of reaction, water was added to the reaction system, and then extracted three times with ethyl acetate, separated and purified by silica column, and characterized by NMR and mass spectrometry. 1 H NMR (500MHz, CDCl 3 )δ7.41-6.82(m,13H),3.79(s,6H),3.67-3.61(m,2H),3.27-3.08(m,4H),2.40(s,1H),1.93(m,2H) ,1.75(m,2H),1.59(s,4H),1.44(m,2H),1.02(s,3H).ESI-MS Calculated for C 33 h 41 N 3 o 5 Na:582.7([M+Na] + ), Found: 582.3.
Embodiment 3
[0039] Synthetic product 4, its synthetic route is as follows:
[0040]
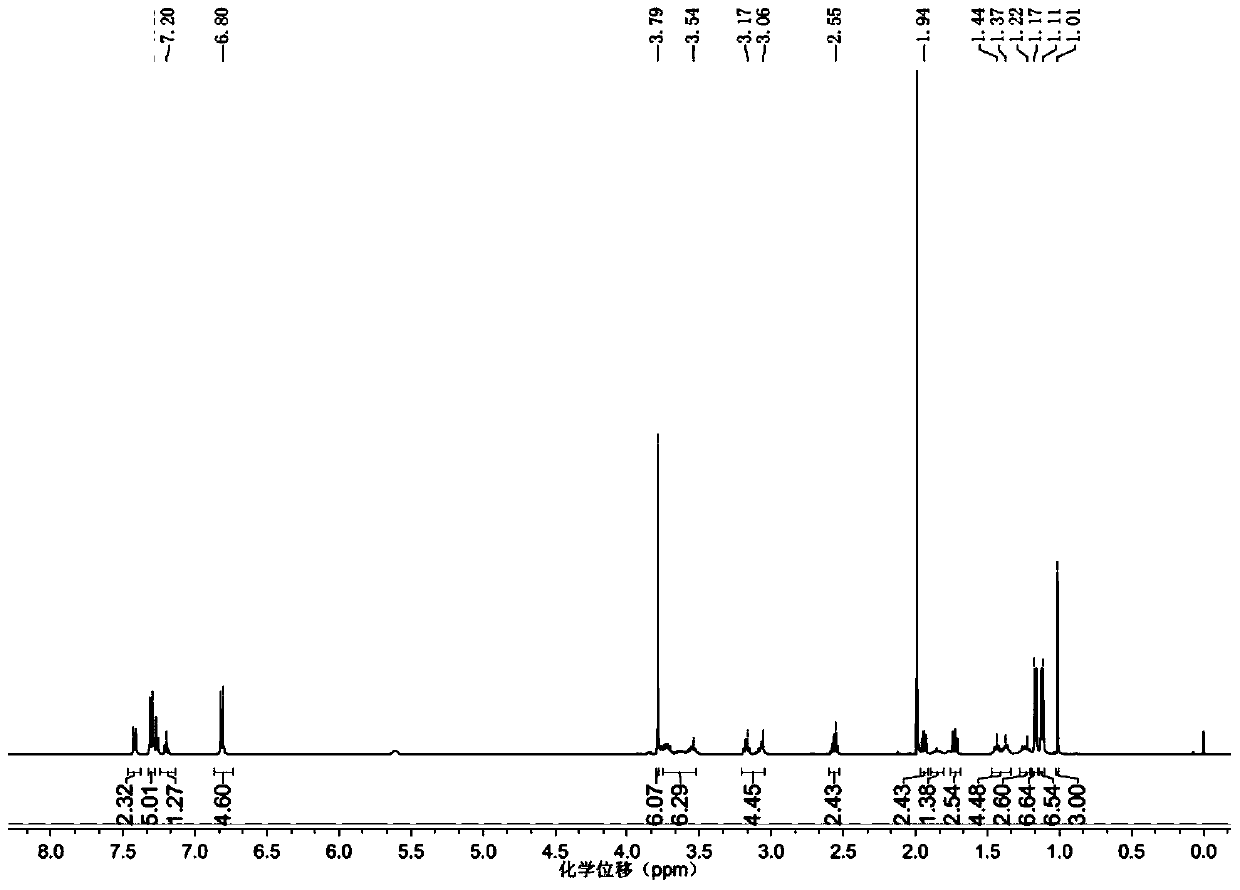
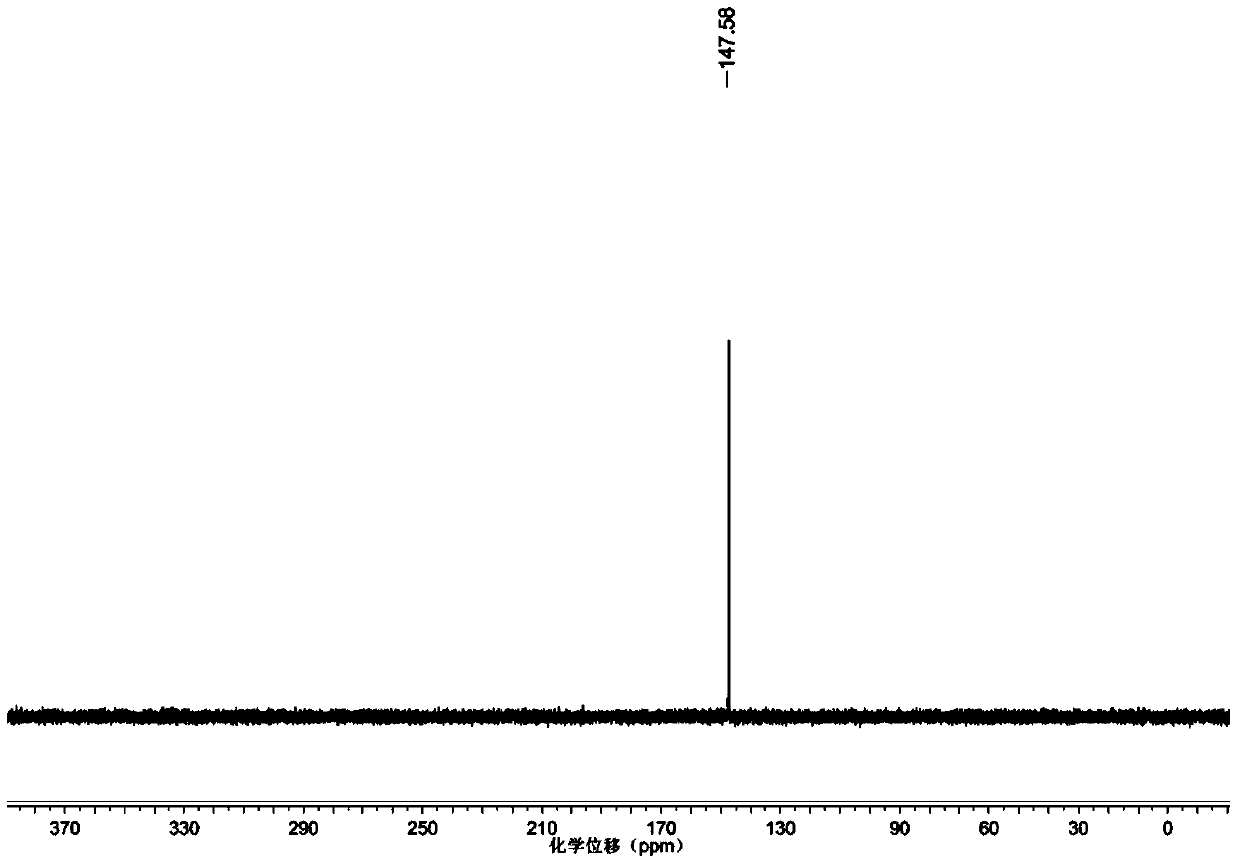
[0041] Add 3 (181.2mg, 0.324mmol) into a round bottom flask, and add 5mL of dichloromethane under nitrogen protection. Add N,N-diisopropylethylamine and 2-cyanoethyl N,N-diisopropylphosphoramidite chloride dropwise under ice bath, react in ice bath for 2h, separate and purify with silica column , NMR and MS characterization, such as figure 1 ,2. 1 H NMR (500MHz, CDCl 3 )δ7.41-6.82(m,13H),3.79(s,6H),3.76-3.5(m,6H),3.27-3.08(m,4H),2.6-2.5(m,4H),1.93(m, 2H),1.85(m,1H),1.72(m,2H),1.4(s,4H),1.2(m,2H),1.15(d,6H),1.12(d,6H),1.02(s,3H ). 31 P NMR (202MHz, CDCl 3 )δ147ppm.ESI-MS Calculated for C 42 h 58 N 5 o 6 PNa:782.9([M+Na] + ), Found: 782.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com