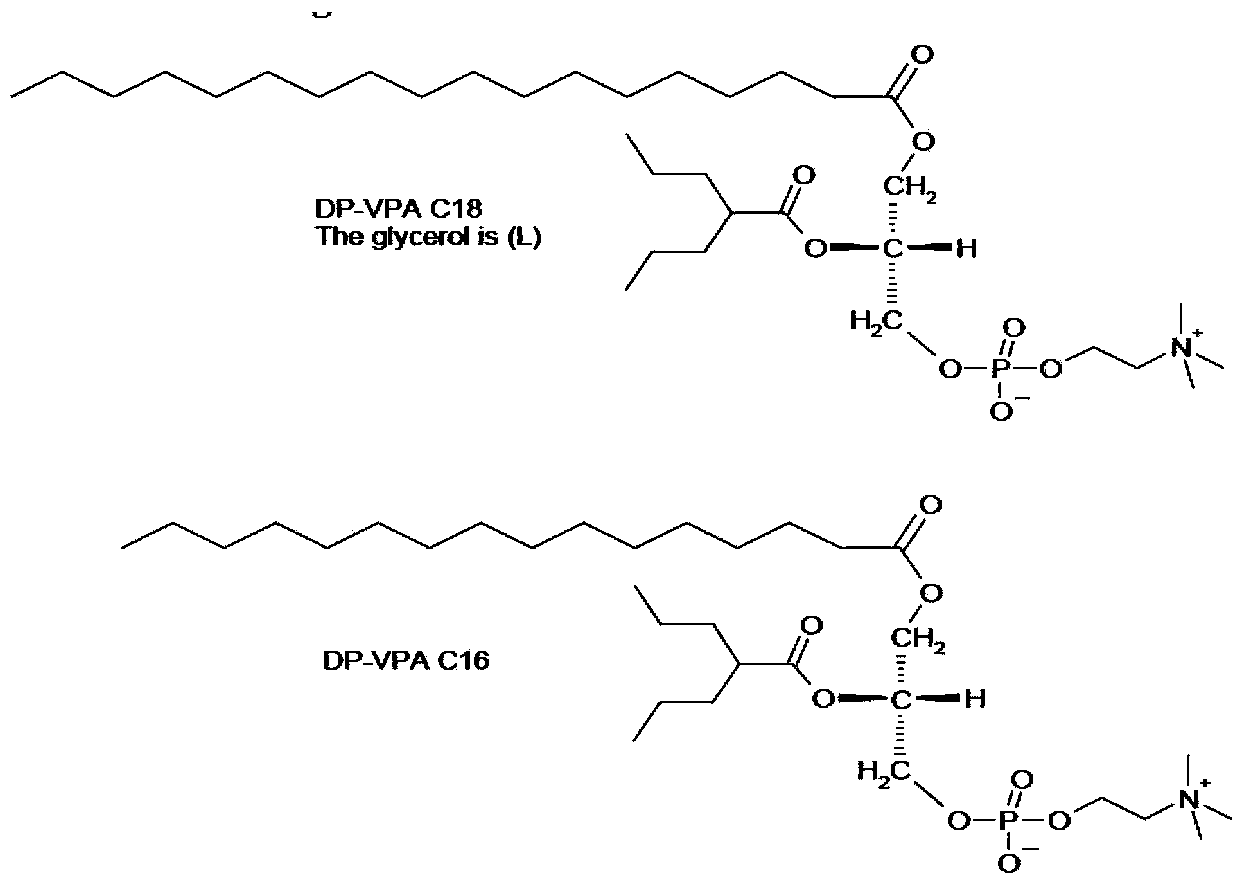
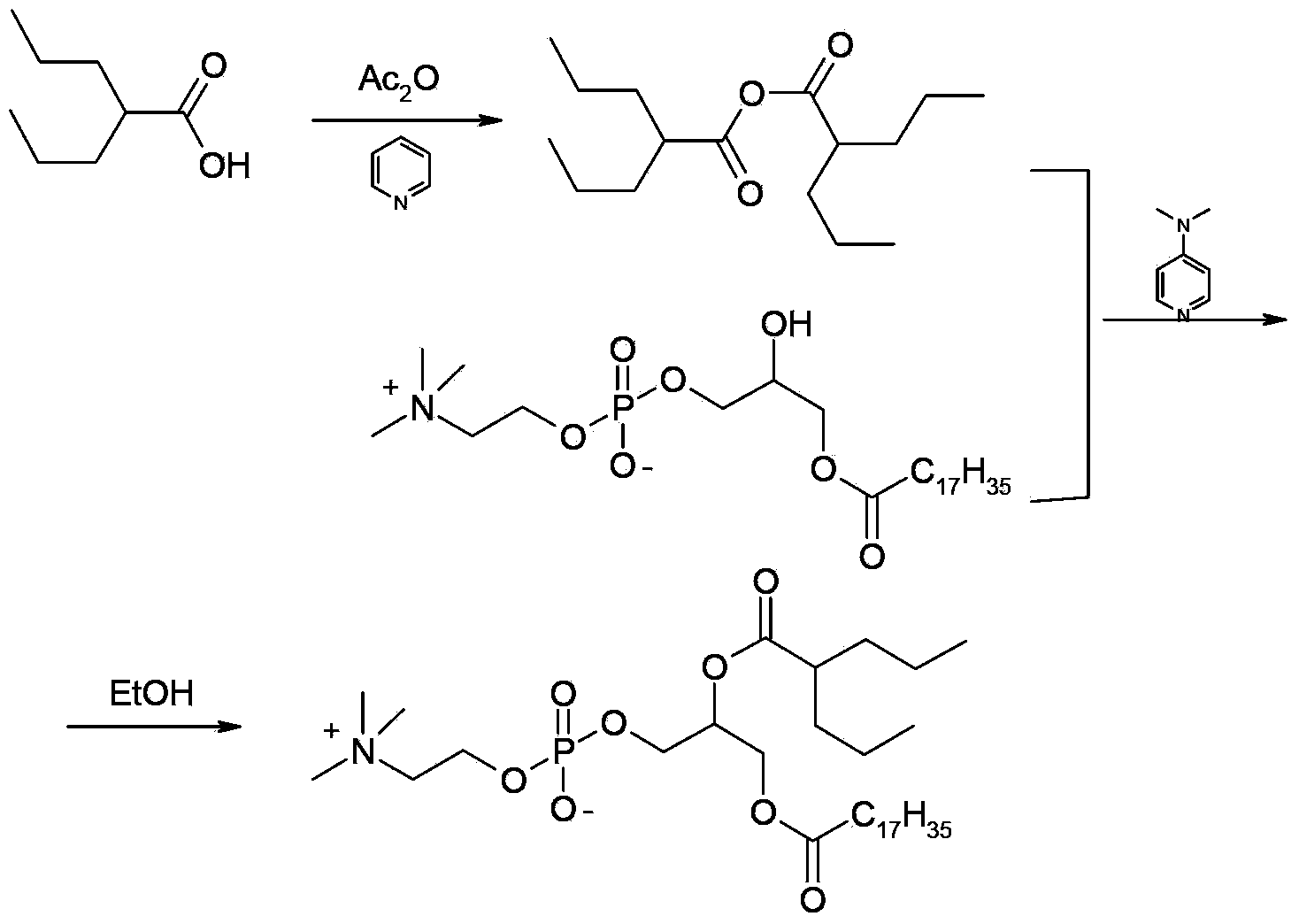
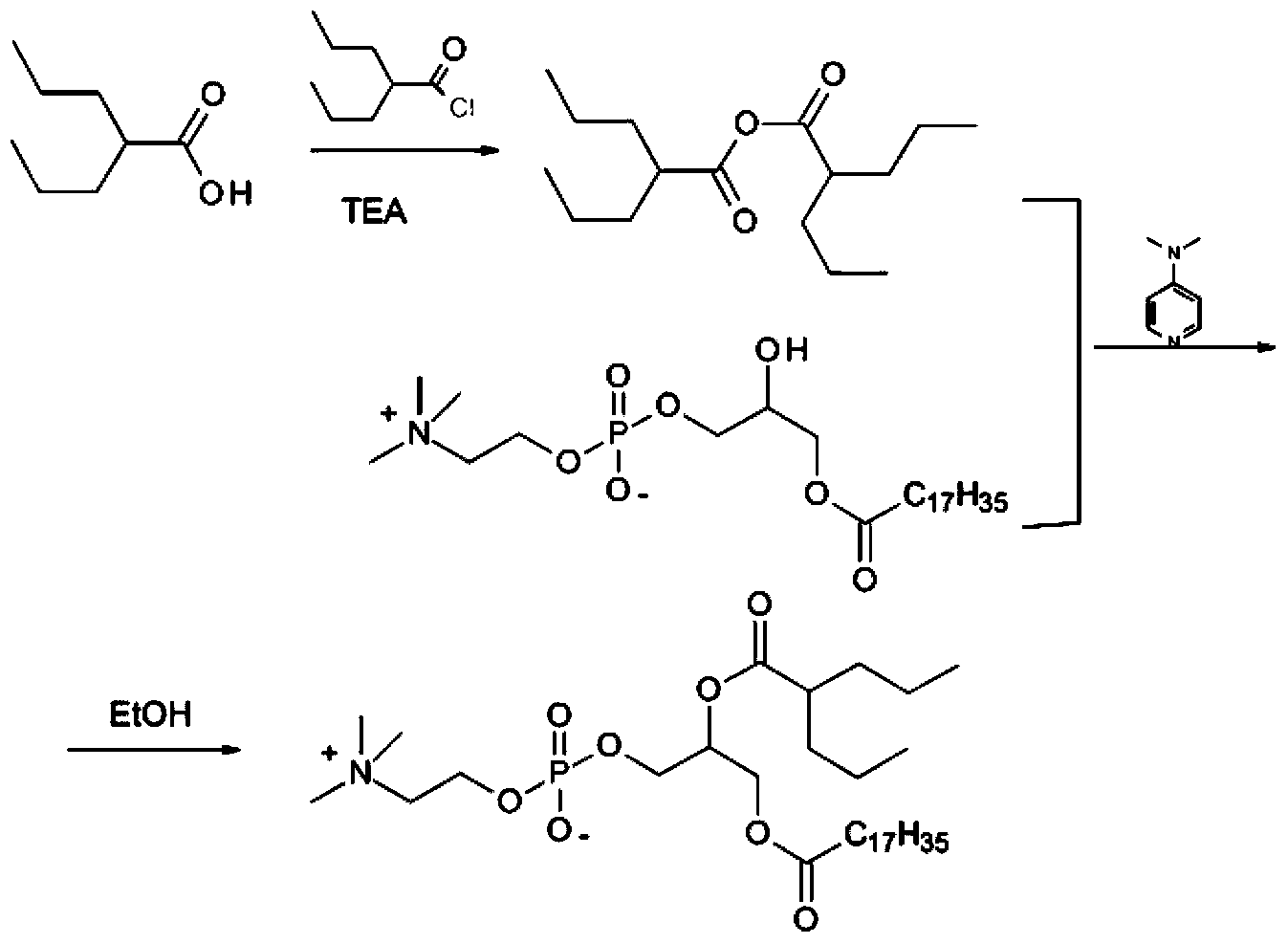
Preparation method of valproic acid phospholipid derivative
A technology of valproic acid phospholipids and derivatives, which is applied in the direction of edible phospholipid composition, food science, protein food ingredients, etc., can solve the problems of high impurity content, low yield, dark product color, etc., and achieve improved product yield and Purity, reduce the content of by-products, and avoid the effect of high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1C 18 -Synthesis of DP-VPA
[0024] (1) Preparation of valproic anhydride
[0025] Step A: add 1.4L of thionyl chloride to a 2L reaction bottle, add 700g of valproic acid dropwise, after the dropwise addition, heat up to 86°C and reflux for 2 hours, remove excess thionyl chloride by rotary evaporation, and the obtained residue is ready for use.
[0026] Step B: In another 5L reaction bottle, add 2L tetrahydrofuran and 491g triethylamine, slowly add 700g valproic acid under stirring, then add the above residue dropwise, after the dropwise addition, stir and react at 10°C for 4h, suction filter, filter cake Washed with tetrahydrofuran, the filtrate was rotary evaporated to remove tetrahydrofuran, the residue was vacuum distilled, and the fraction at 125-127°C / 54Pa was collected to obtain 1089g of valproic anhydride, the yield of valproic anhydride was 82.8%, and the purity was 99.4%.
[0027] (2) C 18 -DP-VPA preparation
[0028] 190g C was added to the 5L rea...
Embodiment 2
[0034] Example 2C 18 -Synthesis of DP-VPA
[0035] (1) Preparation of valproic anhydride
[0036] Step A: Add 1.4L of dichloromethane into a 2L reaction bottle, add 700g of valproic acid dropwise, after the dropwise addition, raise the temperature to 86°C and reflux for 2h, remove excess thionyl chloride by rotary evaporation, and the obtained residue is ready for use.
[0037] Step B: In another 5L reaction bottle, add 2L tetrahydrofuran and 500g pyridine, slowly add 700g valproic acid under stirring, then add the above residue dropwise, after the dropwise addition is completed, stir at 35°C for 1.5h, filter with suction, and filter the cake with THF After washing, the filtrate was rotary evaporated to remove tetrahydrofuran, the residue was vacuum distilled, and the fraction at 125-127°C / 54Pa was collected to obtain 1059 g of valproic anhydride. The yield of valproic anhydride was 80.6%, and the purity was 99.3%.
[0038] (2) C 18 -DP-VPA preparation
[0039] 190g C was ...
Embodiment 3
[0040] Example 3C 18 -Synthesis of DP-VPA
[0041] (1) Preparation of valproic anhydride
[0042] Step A: Add 1.4L of ethane into a 2L reaction flask, add 700g of valproic acid dropwise, after the dropwise addition, raise the temperature to 88°C and reflux for 2h, remove excess thionyl chloride by rotary evaporation, and the obtained residue is ready for use.
[0043] Step B: In another 5L reaction bottle, add 2L tetrahydrofuran and 491g triethylamine, slowly add 700g valproic acid under stirring, then add the above residue dropwise, after the dropwise addition is completed, stir at 25°C for 1h, suction filter, and the filter cake is THF was washed, the filtrate was rotary evaporated to remove THF, the residue was vacuum distilled, and stable fractions at around 125-127°C / 54Pa were collected to obtain 1070 g of valproic anhydride. The yield of valproic anhydride was 81.5%, and the purity was 99.1%.
[0044] (2) C 18 -DP-VPA preparation
[0045] 190g C was added to the 5L rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com