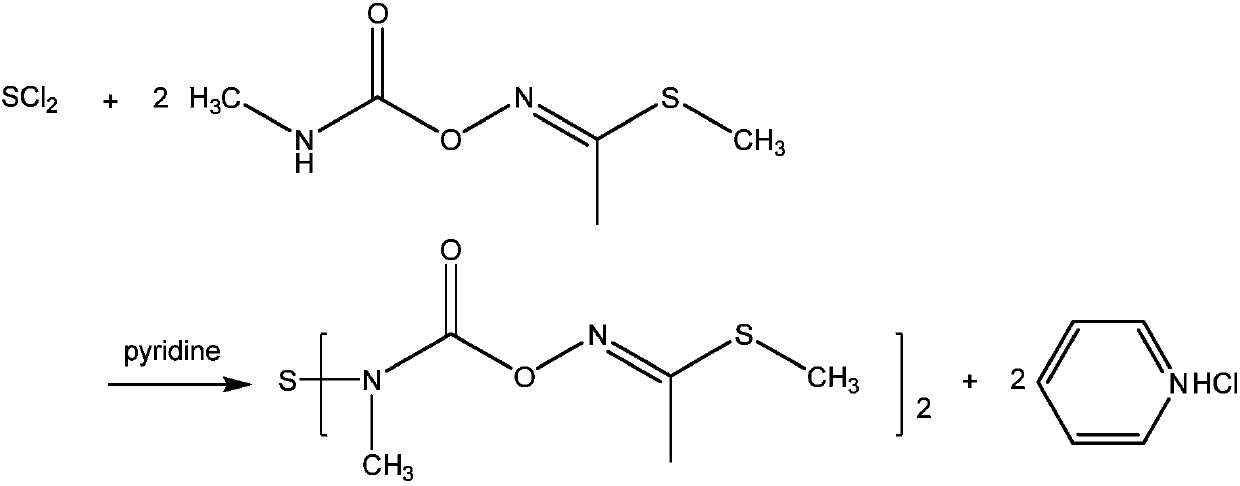
Preparation method of thiodicarb
A technology of thiodimethomyl and methomyl, which is applied in the field of preparation of thiodimethomyl to achieve the effects of reducing self-decomposition, reducing the possibility of high-temperature translocation, and reducing the probability of translocation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 225g of pyridine and 1.62g of 4-dimethylaminopyridine (DMAP) into a 1000mL four-necked flask equipped with mechanical stirring, a thermometer and a constant pressure dropping funnel. Seal the reaction system, cool down to -5-5°C in an ice bath, first drop 1 / 3 of 74.5g of sulfur dichloride (calculated according to 83% mass content, the same below); then gradually increase the temperature to 20°C while simultaneously The pyridine solution of methomyl (162.2g methomyl dissolved in 250g pyridine) and the remaining sulfur dichloride were added dropwise, and the dropwise was completed within 4 hours. After the dropwise addition, the mixture was incubated and reacted at 20° C. for 4 hours. After completion of the reaction, it is detected that the content of methomyl in the reaction solution is 0.3%, and 285g of methanol is added in the reaction solution to quench, stirred and washed at room temperature for 30 minutes, filtered, and the resulting filter residue is dried at ...
Embodiment 2
[0029] Add 225g of pyridine and 1.62g of 4-dimethylaminopyridine (DMAP) into a 1000mL four-necked flask equipped with mechanical stirring, a thermometer, and a constant pressure dropping funnel, seal the reaction system, cool to -5 to 5°C in an ice bath, and drop Add 1 / 3 of 72.0g of sulfur dichloride, and then gradually add the pyridine solution of methomyl (162.2g of methomyl dissolved in 250g of pyridine) and the remaining sulfur dichloride , finished dripping within 4 hours. After the dropwise addition, the mixture was incubated and reacted at 20° C. for 4 hours. After completion of the reaction, it is detected that the methomyl content in the reaction solution is 0.29%. Add 285g of methanol to the reaction solution to quench, stir and wash at room temperature for 30 minutes, filter, and the resulting filter residue is dried at 50°C, which is thiodicarb product.
[0030] After testing, the product quality was 155.4g, the mass percentage of thiodicarb was 97.5%, the yield ...
Embodiment 3
[0032] Add 225g of pyridine and 1.62g of 4-dimethylaminopyridine (DMAP) into a 1000mL four-necked flask equipped with mechanical stirring, a thermometer, and a constant pressure dropping funnel, seal the reaction system, cool to -5 to 5°C in an ice bath, and drop Add 1 / 3 of 67.01g of sulfur dichloride, and then gradually add the pyridine solution of methomyl (162.2g of methomyl dissolved in 250g of pyridine) and the remaining sulfur dichloride , finished dripping within 4 hours. After the dropwise addition, the mixture was incubated and reacted at 20° C. for 4 hours. After completion of the reaction, it is detected that the content of methomyl in the reaction solution is 0.28%. In the reaction solution, 285g of methanol is added to quench, stirred and washed at room temperature for 30 minutes, filtered, and the resulting filter residue is dried at 50°C, which is thiodicarb product.
[0033] After testing, the product quality was 154.7g, the mass percentage of thiodicarb was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com