Method for synthesizing phenylalanine esterified bagasse xylan-g-CHMA (cyclohexyl methacrylate) with anti-tumor activity
A technology of phenylalanine ester and synthesis method, which is applied in the field of synthesizing anticancer activity phenylalanine esterified bagasse xylan-g-CHMA, can solve the problems of being insoluble in water, restricting application and the like, and achieves enhanced biological The effect of activity, high grafting rate and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
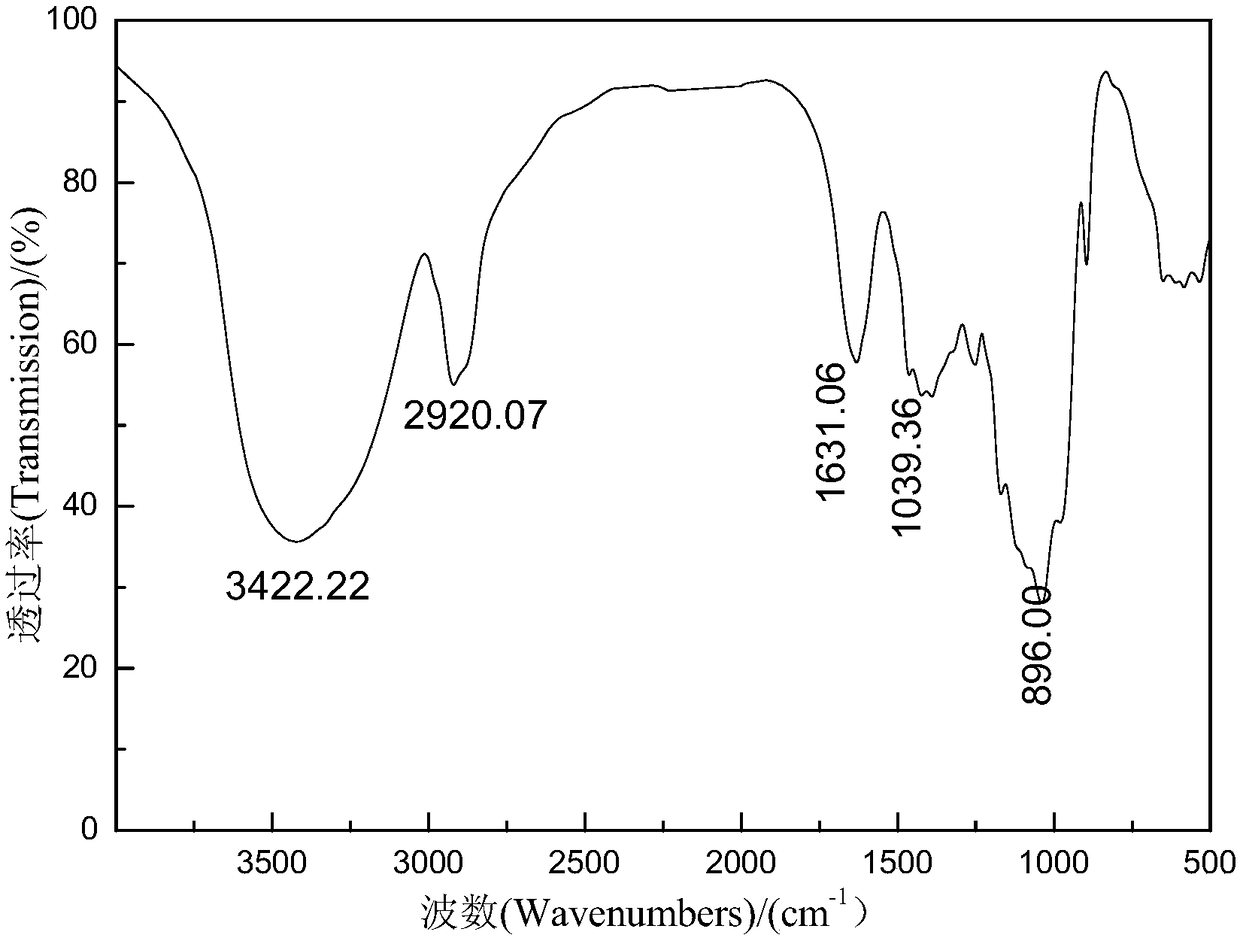
Image
Examples
Embodiment
[0038] (1) Put 5 g of bagasse xylan in a constant-temperature vacuum oven at 60° C. and dry for 24 hours to constant weight to obtain dry-based bagasse xylan.
[0039] (2) Measure 10 mL of analytically pure N-methylimidazole and 12 mL of analytically pure chloroethanol into a 250 mL four-neck flask, stir and react at 70° C. for 24 hours, and cool down to room temperature.
[0040] (3) Place the material obtained in step (2) in a refrigerator at 5°C for 10 hours to precipitate a precipitate, wash it with 15mL of analytically pure ethyl acrylate, and filter it with suction three times, put the filter cake in a watch glass, and place it in a constant vacuum at 45°C Dry in a drying oven for 24 hours to obtain 1-(2-hydroxyethyl)-3-methylimidazole chloride ([Hemim]Cl), a solid ionic liquid at room temperature.
[0041] (4) Weigh 0.3g of ammonium persulfate into a 50mL beaker, add 15mL of analytically pure dimethyl sulfoxide (DMSO), stir at 20°C to obtain a mixed solution of initiato...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com