Purification method of acetyl pullulan polysaccharide folate conjugate and preparation method of nanometer particles thereof
A technology of pullulan polysaccharide folic acid and pullulan polysaccharide, which is applied in the directions of non-active ingredients such as medical preparations, pharmaceutical formulations, powder delivery, etc., to achieve the effects of stable structure, controllable preparation conditions and small particle size distribution range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Precipitation and purification method of folic acid acetyl pullulan conjugate:
[0025] Dissolve 1g (2.27mmol) of folic acid in 15ml of dimethyl sulfoxide (DMSO), dropwise add 5 drops of triethylamine, stir to dissolve completely, add 0.9g (4.4mmol) of N,N'-dicyclohexylcarbodiethylene Amine (DCC), 0.25g (2.05mmol) 4-dimethylaminopyridine (DMAP), stirred for 1 hour, then added 0.615g of acetyl pullulan dissolved in 6.15ml of DMSO, and reacted in the dark for 5 days under electromagnetic stirring. Remove the DCU precipitate by filtration, add the filtrate dropwise to 250ml of absolute ethanol solution, centrifuge (8000 rpm, 4°C, centrifuge for 15min) to collect the yellow precipitate, dialyze in sodium carbonate buffer for 24h, dialyze in deionized water for 48h, freeze-dry or vacuum After drying, a yellow powder is obtained, which is a folic acid-coupled acetyl pullulan derivative.
[0026] The proton magnetic spectrum ( 1 H-NMR) is basically consistent with...
Embodiment 2
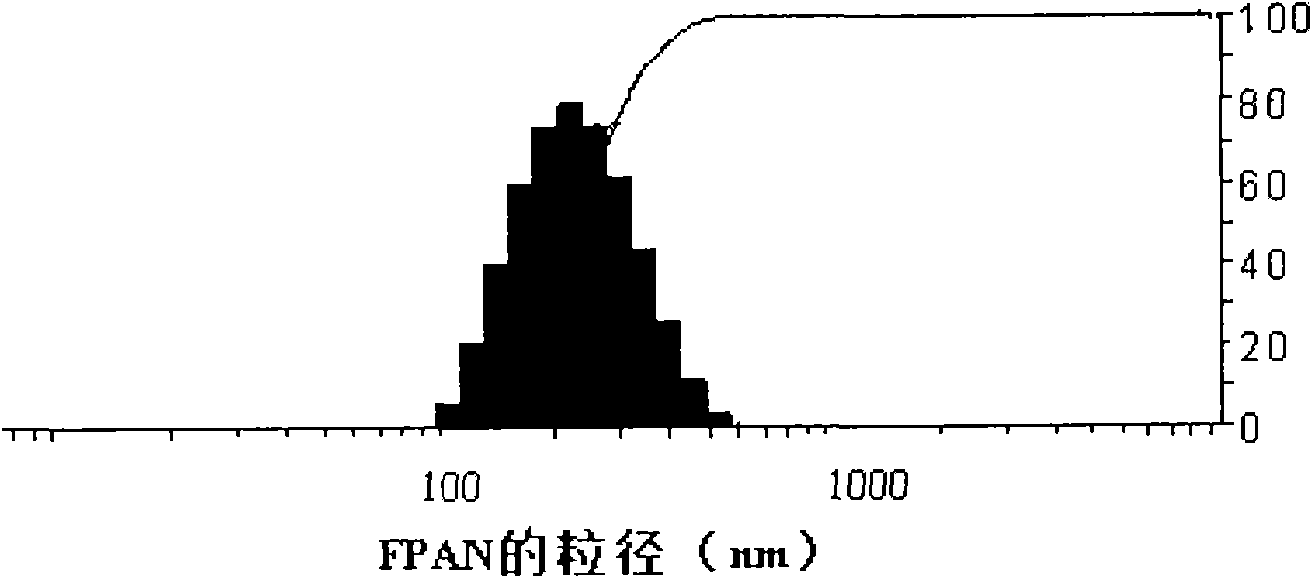
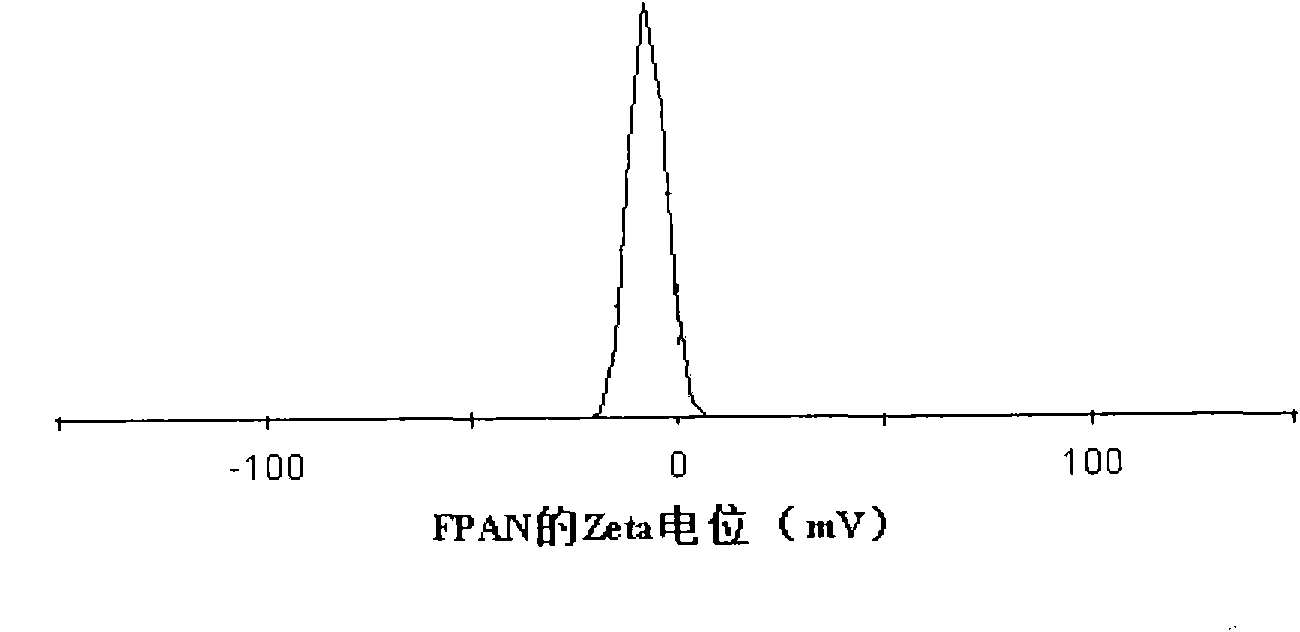
[0027] Embodiment 2: the preparation of FPA nanoparticle
[0028] Accurately weigh 48 mg of acetyl pullulan polysaccharide folic acid conjugate and dissolve it in 4.8 ml of dimethyl sulfoxide at a concentration of 10 mg / ml. Transfer the solution to a dialysis bag for dialysis. The molecular weight cut-off of the dialysis bag is 14000 Da, and the dialysate is For deionized water, change the water every 1-2 hours at the beginning, change it 4 times, and then change the water every 4-6 hours, for a total of 48 hours of dialysis. The final solution is a solution with yellow opalescence, which is transferred to a graduated centrifuge tube, and after 100W ultrasound for 3 minutes, it is the final nanoparticle suspension, and the final concentration is the volume of the added material (48mg) and the final nanoparticle solution ( 12ml) than 4mg / ml. According to the needs of the experiment, it can be diluted with water or other solutions to the target concentration, or the nanoparticl...
Embodiment 3
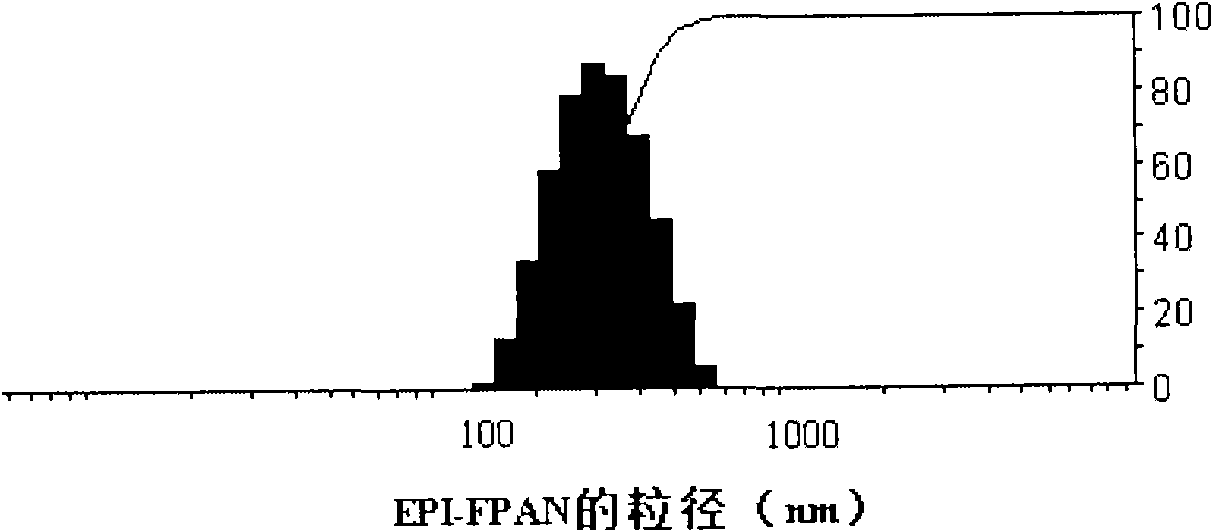
[0029] Embodiment 3: the preparation of the FPA nanoparticle that packs epirubicin
[0030] Accurately weigh 40 mg of acetyl pullulan folic acid conjugate, and dissolve it in 3.2 ml of dimethyl sulfoxide to make the concentration 12.5 mg / ml. Dissolve 10 mg of epirubicin in 2 ml of dimethyl sulfoxide at a concentration of 5 mg / ml, add 4.9 μl of triethylamine (2 times the molar amount), and stir at room temperature for 12 hours in the dark to dehydrochloride the epirubicin. Mix the drug / material solution at a volume ratio of 4:1, transfer the solution to a dialysis bag for dialysis, the molecular weight cut-off of the dialysis bag is 10,000 Da, and the dialysate is deionized water. Change the water once every 1 to 2 hours, and change it 4 times Afterwards, the water was changed every 4 to 6 hours for a total of 48 hours of dialysis. The final solution is an orange-red opalescent solution, which is transferred to a graduated centrifuge tube. After 100W ultrasonication for 3 minu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com