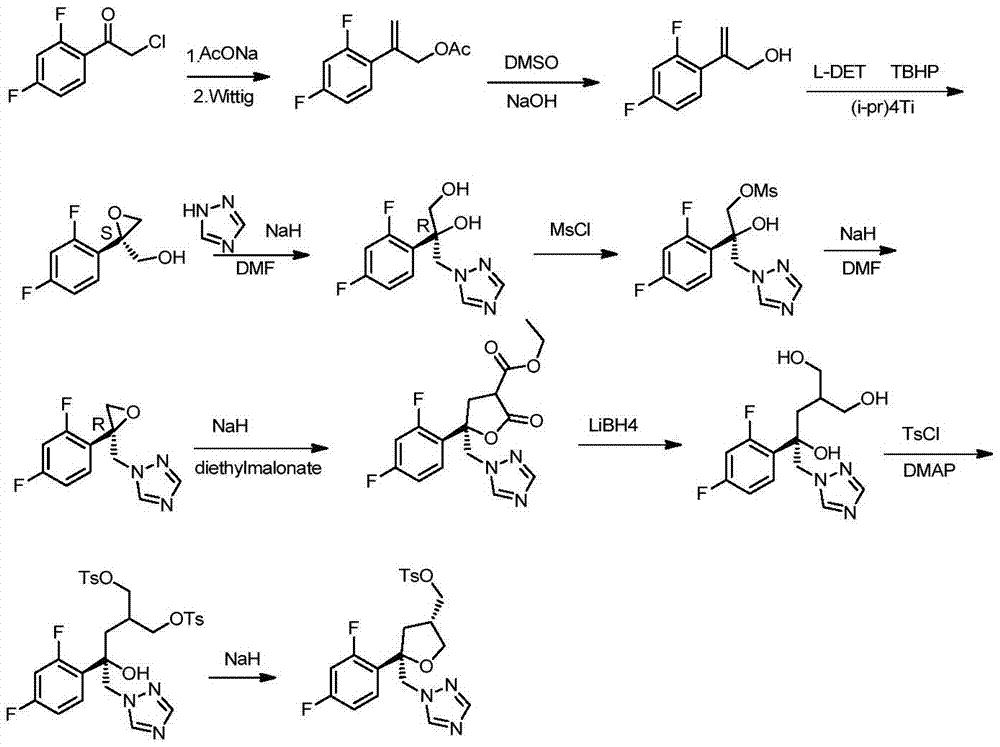
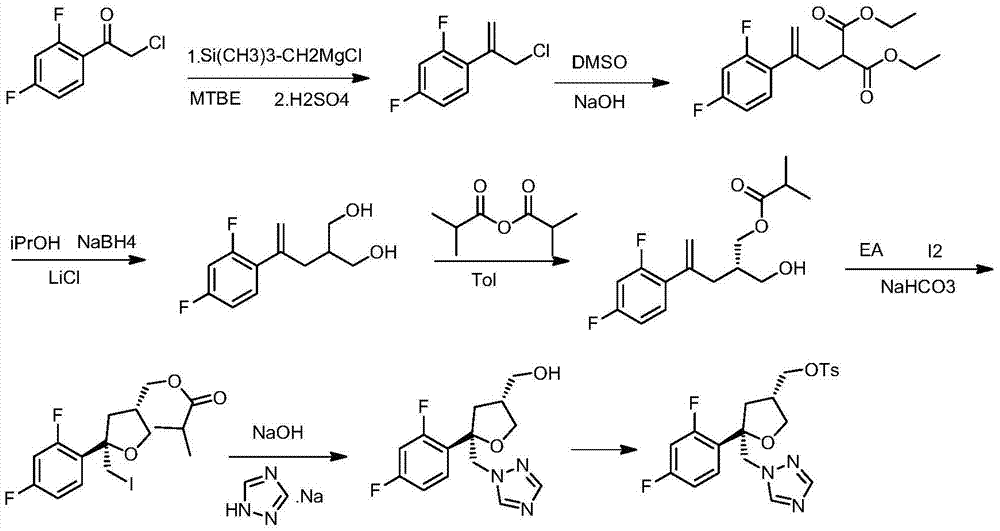
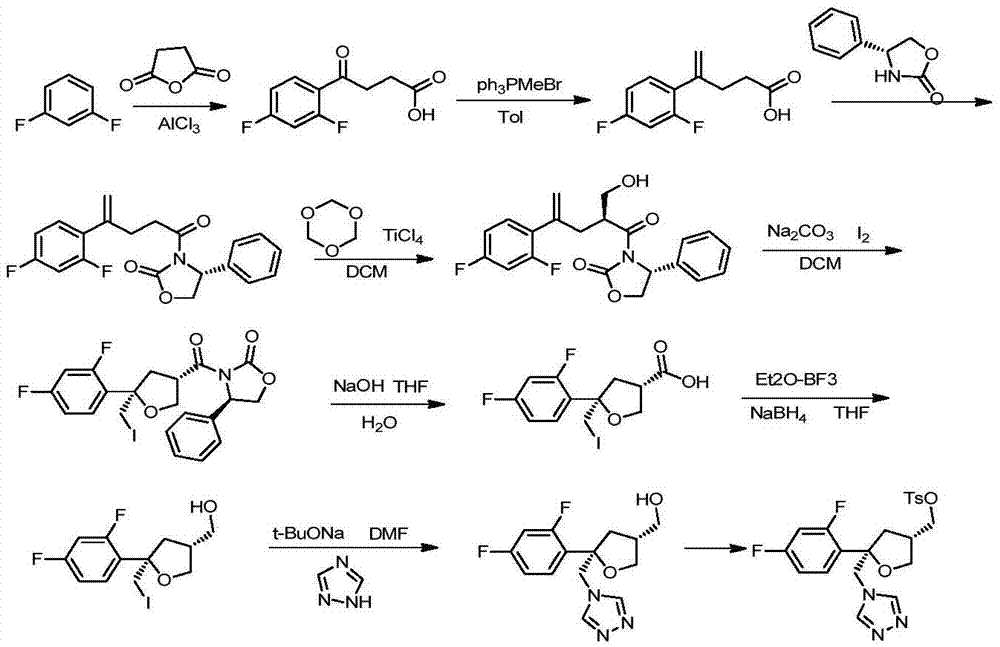
Preparation method of posaconazole main ring
A technology of posaconazole main ring and sodium triazole, which is applied in the field of organic synthesis, can solve problems affecting yield, etc., and achieve the effects of stable reaction, safe operation and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 prepares compound F
[0020] Referring to the method published by India MSN Laboratories Limited on US2014343285A1, compound F was prepared using m-difluorobenzene as a starting material, and the following steps were followed:
[0021] 1. Add 450g (1eq) m-difluorobenzene and 360g (1eq) succinic anhydride to a 10L three-necked flask at room temperature, add 4.5L dichloromethane (DCM), and then add 1200g (2.5eq) AlCl in batches 3 , the temperature should not exceed 30°C, and the color will slowly turn orange-red. After adding, react at room temperature for 4h, and the reaction is complete. Pour the reaction liquid into 3.6L 6NHCl slowly, and stir slowly, the temperature should not exceed 30°C. The organic phase was separated, and the aqueous phase was extracted with 1L*3 DCM. The combined organic phases were washed with 4.5L*2 saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure. Add 450ml of DCM and 4500...
Embodiment 2
[0027] Embodiment 2 prepares target compound J with compound F
[0028] Follow the steps below:
[0029] 1) Take 180g (1eq) of compound F prepared in Example 1 and add it into 1.8L of methanol, under nitrogen protection, cool down to 0-10°C, add 175g (3eq) of thionyl chloride (SOCl) dropwise 2), then reflux for 12 hours, the reaction solution was concentrated to dryness under reduced pressure, 900ml of water was added to the residue, extracted with 300ml*3 ethyl acetate (EA), combined EA, washed with 300ml of saturated sodium carbonate, washed with 300ml of water, washed with 300ml of saturated brine, Dry over anhydrous sodium sulfate and concentrate to dryness under reduced pressure to obtain 190 g of compound G, Y>100%, pur=96.2%.
[0030] 2) Add 150g (1eq) of compound G to 1.5LDMF, add 178.6g (5eq) of sodium triazole and 38ml of 1,3-dimethylpropylene urea (DMPU), heat to 100-110°C and stir for 24 hours. Cool down to room temperature, add 1.5L of water to the reaction solu...
Embodiment 3
[0032] 4) Add 60g (1eq) of compound I to 600ml of DCM, then add 24.6g (1.2eq) of triethylamine (TEA) and 2.5g (0.1eq) of 4-dimethylaminopyridine (DMAP). Under nitrogen protection, lower the temperature to 0°C and add 44g (1eq) 4-methylbenzenesulfonyl chloride (TsCl) at one time (the amount of TEA needs to be more than TsCl) to rise to room temperature and react overnight. The reaction solution is washed with 200ml 1N HCl, and then saturated with 100ml of water Washed with sodium bicarbonate, washed with 100ml saturated brine, Na 2 SO 4 Dry, filter, and spin dry to obtain a yellow oil, add 360ml of isopropanol, stir to dissolve at 50-60°C, add dropwise 360ml of n-heptane, drop to room temperature and stir for 1.5 hours, filter with suction, and wash with 100ml of n-heptane , to obtain white solid compound J75.1g, Y=82.2%, pur=98.7%. Embodiment 3 prepares target compound J with compound F
[0033] Follow the steps below:
[0034] 1) Take 180g (1eq) of compound F prepared in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com