Preparation method of mild diazomethane derivative
A technology for diazomethane and derivatives, applied in the field of preparation of diazomethane derivatives, can solve the problems of harsh conditions, limited practicability, unfavorable subsequent reactions, etc., and achieves stable chemical properties, simple synthesis operations, and cheap and easy raw materials. the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The invention provides a kind of preparation method of diazomethane derivative, comprising the following steps:
[0073] (1) Carry out substitution reaction with EWG substituted benzenesulfonyl chloride having the structure shown in formula I and hydrazine hydrate, obtain the EWG substituted benzenesulfonyl hydrazide having the structure shown in formula II;
[0074]
[0075] The EWG is an electron-withdrawing group; the electron-withdrawing group is 2-nitro, 4-nitro, 2,4-dinitro, 2-trifluoromethyl, 2-cyano, 2,3, One of 4,5,6-pentafluoro, 2,3,4,6-tetrafluoro, 2,4,6-trifluoro, 3,4,5-trifluoro;
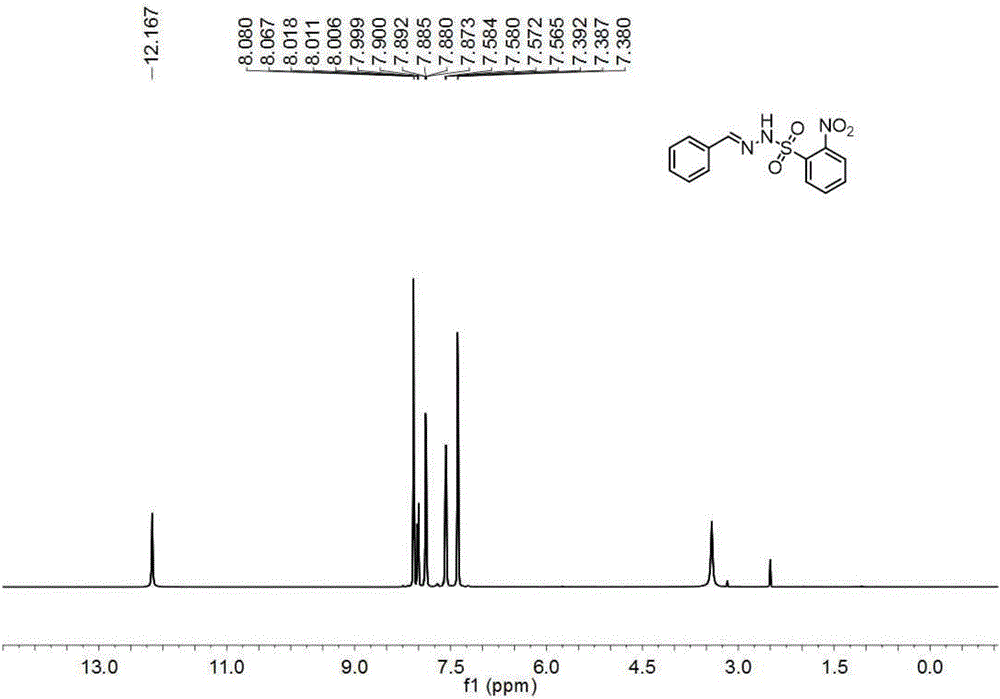
[0076] (2) Condensing the EWG substituted benzenesulfonyl hydrazide with the structure shown in formula II obtained in the step (1) with the aldehyde or ketone with the structure shown in formula III to obtain the EWG substituted benzene with the structure shown in formula IV Sulfonylhydrazone;
[0077]
[0078] The R 1 is aryl, heteroaryl, alkyl, alkenyl or alkynyl; R ...
Embodiment 1
[0110] (1)
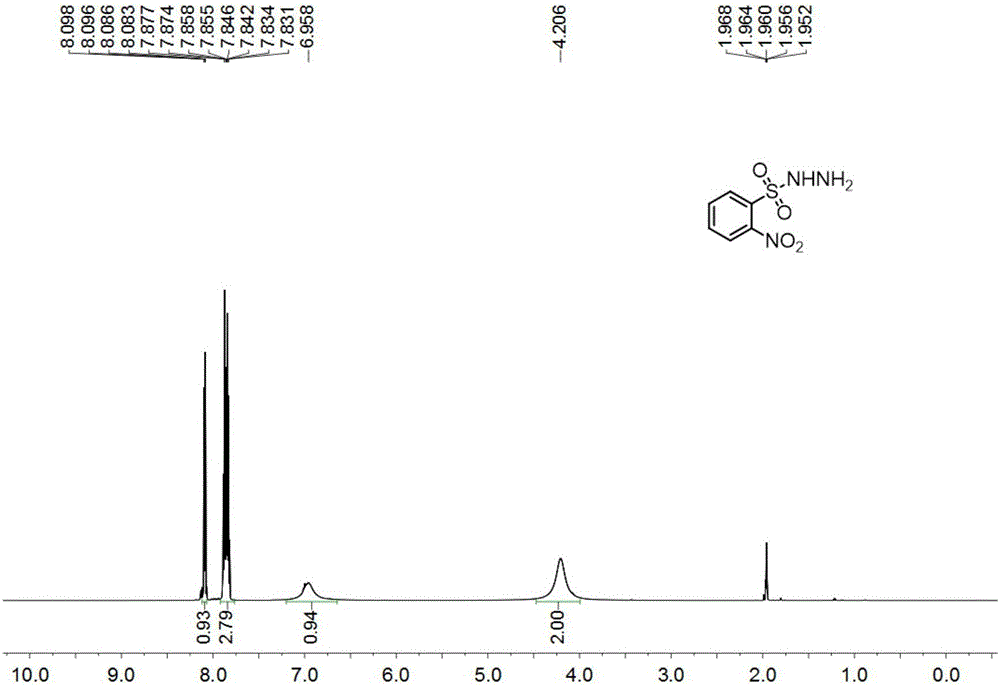
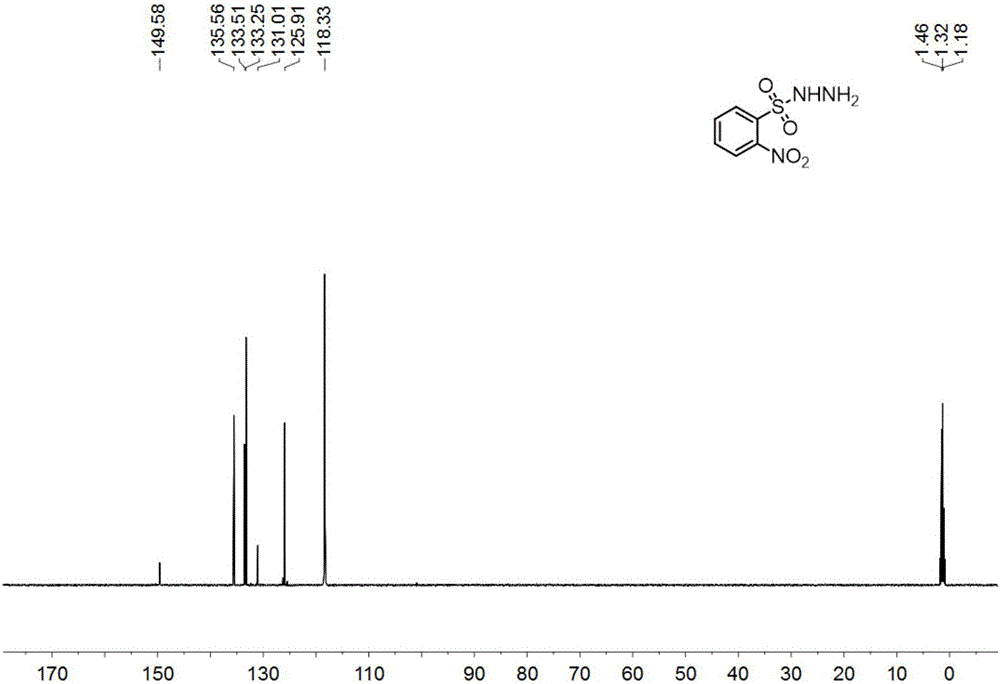
[0111] Under nitrogen, add o-nitrobenzenesulfonyl chloride (11.0g, 50mmol) and tetrahydrofuran (100mL) into a 250mL reaction flask, stir until dissolved, cool to 0°C in an ice-water bath, add hydrazine hydrate (6.1mL, 125mmol) dropwise , dropwise, react at 0°C until o-nitrobenzenesulfonyl chloride disappears as monitored by thin-layer chromatography, pour the reaction solution into ethyl acetate (200mL), separate the layers, wash the organic phase with 10% aqueous sodium chloride, dry, and extract Filter, drop in 800ml petroleum ether, have o-nitrobenzenesulfonyl hydrazide to separate out gradually, filter, recrystallize, vacuum-dry to obtain yellow solid (9.1g, yield 83.9%), i.e. o-nitrobenzenesulfonyl hydrazide, structure Characterized as figure 1 , figure 2 and the data shown below.
[0112] Yellow solid, m.p.100-101°C; 1 H-NMR (600MHz, CD 3 CN)δ8.10-8.08(m,1H),7.88-7.83(m,3H),6.96(s,1H),4.21(s,2H); 13 C-NMR (150MHz, CD 3 CN) δ149.6, 135.6, 133.5, 133.3...
Embodiment 2
[0121] (1) Under nitrogen, add o-trifluoromethylbenzenesulfonyl chloride (12.2g, 50mmol) and tetrahydrofuran (100mL) into a 250mL reaction flask, stir until dissolved, cool to -10°C, add hydrazine hydrate (6.1 mL, 125mmol). After dropping, react at -10°C until o-trifluoromethylbenzenesulfonyl chloride disappears as monitored by thin-layer chromatography, pour the reaction solution into ethyl acetate (200 mL), separate the layers, wash the organic phase with 10% aqueous sodium chloride solution, and dry. Suction filtration, drop in 800ml petroleum ether, have o-trifluoromethylbenzenesulfonyl hydrazide to separate out gradually, filter, recrystallize, vacuum-dry to obtain white crystal and be o-trifluoromethylbenzenesulfonyl hydrazide (9.7g, yield 80.7 %), its structure is characterized by Figure 7 , Figure 8 and the data shown below.
[0122] White crystals, m.p.111-112°C; 1 H-NMR (600MHz, CD 3 CN)δ8.20-8.19(m,1H),7.99-7.98(m,1H),7.86-7.83(m,2H),6.77(s,1H),3.87(s,2H); 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com