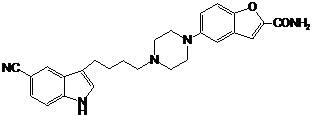
Preparation method of vilazodone
The technology of vilazodone and nitro group is applied in the field of improved preparation of antidepressant drug vilazodone, can solve the problems of unobtainable reagents, complicated operation, low yield and the like, and achieves convenient industrial production and simple post-processing. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
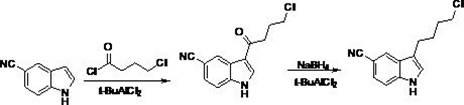
[0060] Example 1 Preparation of compound 1-benzenesulfonyl-1H-indole-5-carbonitrile
[0061] Add 60% sodium hydrogen (5.6g, 0.14mol) and tetrahydrofuran (200ml) into a 500mL three-neck reaction flask, and then lower the temperature to below 0°C. Add 5-cyanindole (20 g, 0.14 moL) slowly, and stir for 20 minutes after the degassing is complete. Slowly add benzenesulfonyl chloride (49.5 g, 0.28 mol). After the addition is complete, slowly raise the temperature to 40°C for reaction. TLC monitors that the reaction of the raw materials is complete. The reaction solution was extracted with saturated ammonium chloride solution, the aqueous phase was extracted twice with ethyl acetate, the organic phases were combined, washed with water, and dried overnight. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain compound 1-benzenesulfonyl-1H-indole-5-carbonitrile (39 g, 0.14 moL).
[0062] The structure of the compound obtained in this example is...
Embodiment 2
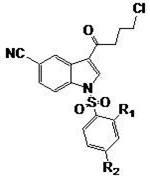
[0064] Example 2 Preparation of compound 3-(4-chlorobutyryl)-1-(benzenesulfonyl)-1H-indole-5-carbonitrile
[0065] Aluminum trichloride (56.7g, 0.43mol) and dichloromethane (800ml) were added to a 1L three-neck reaction flask, and 4-chlorobutyryl chloride (61.2g, 0.43mol) was slowly added at room temperature, and stirred for 20 minutes. A solution of 1-benzenesulfonyl-1H-indole-5-carbonitrile (40 g, 0.14 mol) in dichloromethane (200 ml) was slowly added. After the addition was complete, the stirring reaction was continued, and the reaction of the raw materials was monitored by TLC. The reaction solution was extracted with ice water, the aqueous phase was extracted twice with dichloromethane, the organic phases were combined, washed with water, and dried overnight. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain the compound 3-(4-chlorobutyryl)-1-(benzenesulfonyl)-1H-indole-5-carbonitrile (39.8g, 0.1moL).
[0066] The structure of ...
Embodiment 3
[0068] The preparation of embodiment 3 compound 3-(4-chlorobutyl)-1-(benzenesulfonyl)-1H-indole-5-carbonitrile
[0069] Add 3-(4-chlorobutyryl)-1-(benzenesulfonyl)-1H-indole-5-carbonitrile (1g, 2.6mmol), triethylsilane (0.9g, 7.8 mmol) and trifluoroacetic acid (20ml), the temperature was raised to 50°C, and the reaction was stirred. TLC monitored the completion of the raw material reaction. The reaction solution was extracted with 2N hydrochloric acid solution. The aqueous phase was extracted twice with ethyl acetate. The organic phases were combined, washed with water, and dried overnight with anhydrous sodium sulfate. Filtration, the filtrate was concentrated under reduced pressure until a solid precipitated, stirred for 0.5 hours, and filtered to obtain the compound 3-(4-Chlorobutyl)-1-(benzenesulfonyl)-1H-indole-5-carbonitrile (0.9g, 2.4mmol).
[0070] The structure of the compound obtained in this example is , Yield: 93%.
[0071] 1 H NMR (CDCl3)δ8.4(d, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com