Preparation method of ceftaroline fosamil intermediate parent nucleus
A technology for ceftaroline fosamil and intermediates is applied in the field of preparation of ceftaroline fosamil intermediate parent nucleus, and can solve problems such as troublesome quality control of intermediates, high toxicity of methanesulfonyl chloride, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
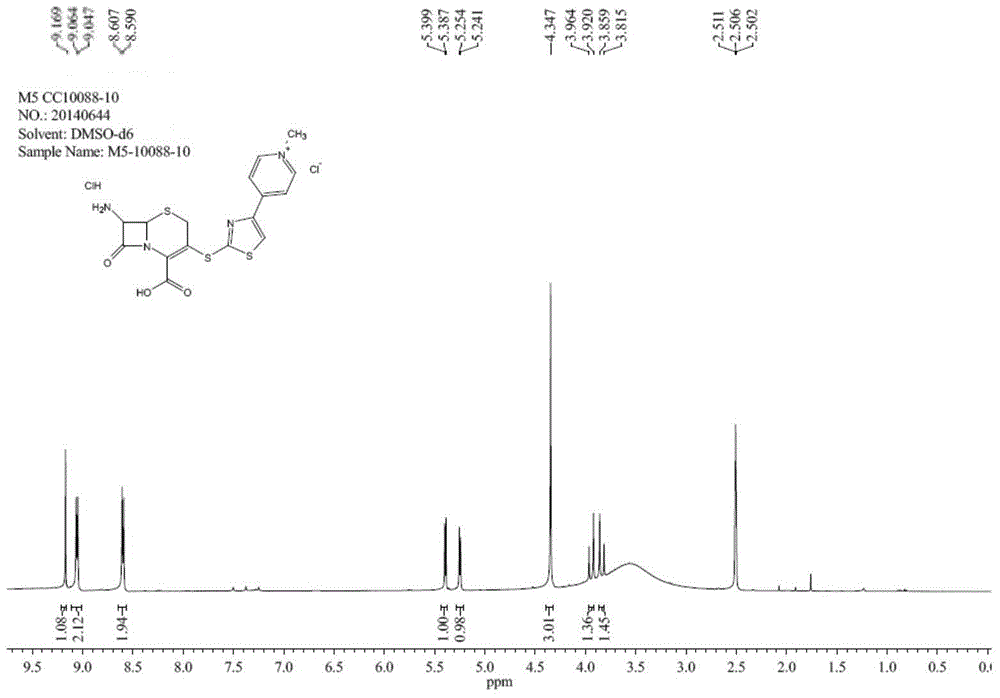
[0033] The invention provides a kind of preparation method of ceftaroline axetil intermediate core, comprising the following steps: A) reacting 3-hydroxycephalosporin and activating reagent under the condition that acid-binding agent and organic solvent exist, obtain activated intermediate; the activating reagent is p-toluenesulfonyl chloride, benzenesulfonyl chloride, 4-nitrobenzenesulfonyl chloride, trifluoroacetic anhydride or trifluoromethanesulfonic anhydride; B) the activated intermediate and 4-( 4-pyridyl)-2-mercaptothiazole reaction to obtain an intermediate shown in formula (I); C) reacting the intermediate shown in formula (I) with a quaternizing agent to obtain a pyridinium salt; D) deprotecting the pyridinium salt to obtain the ceftaroline axetil intermediate nucleus shown in formula (II);
[0034]
[0035] The present invention uses 3-hydroxycephalosporin as a raw material, and the raw material is easy to get; the activating reagent is preferably p-toluenesulfo...
Embodiment 1
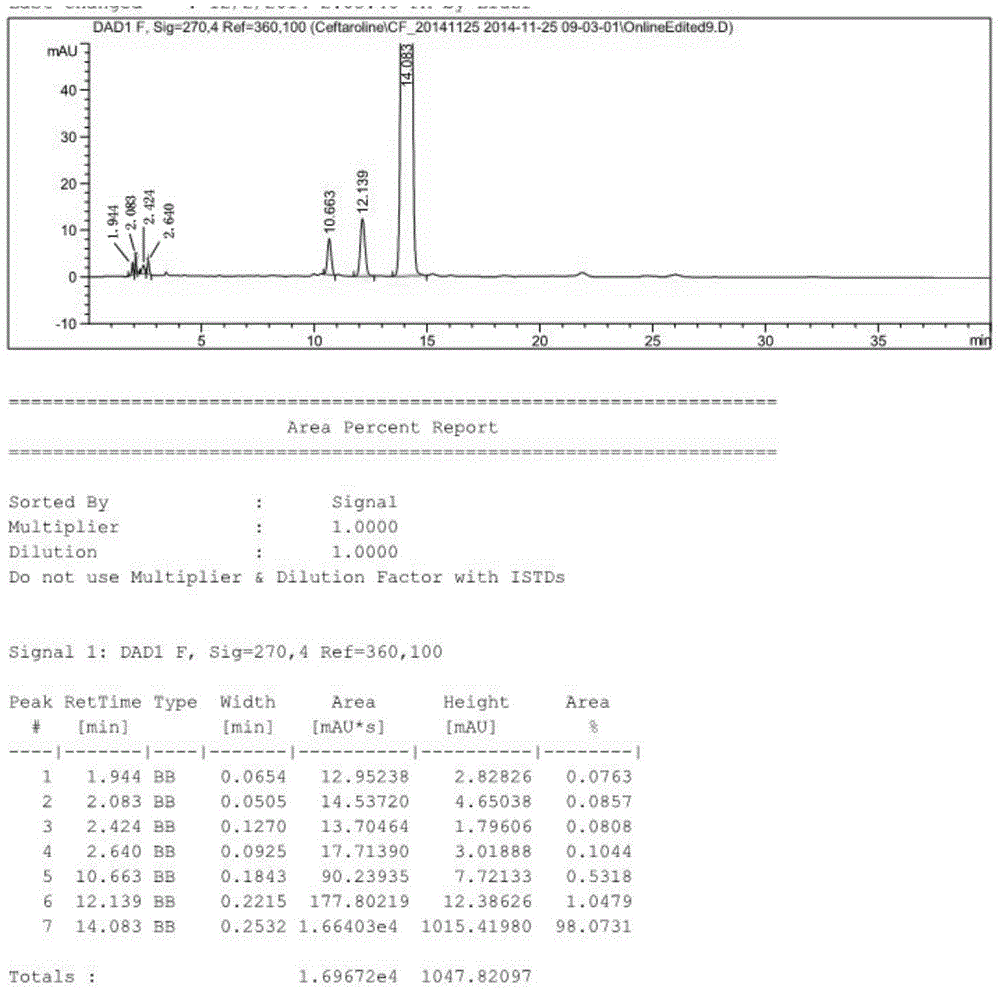
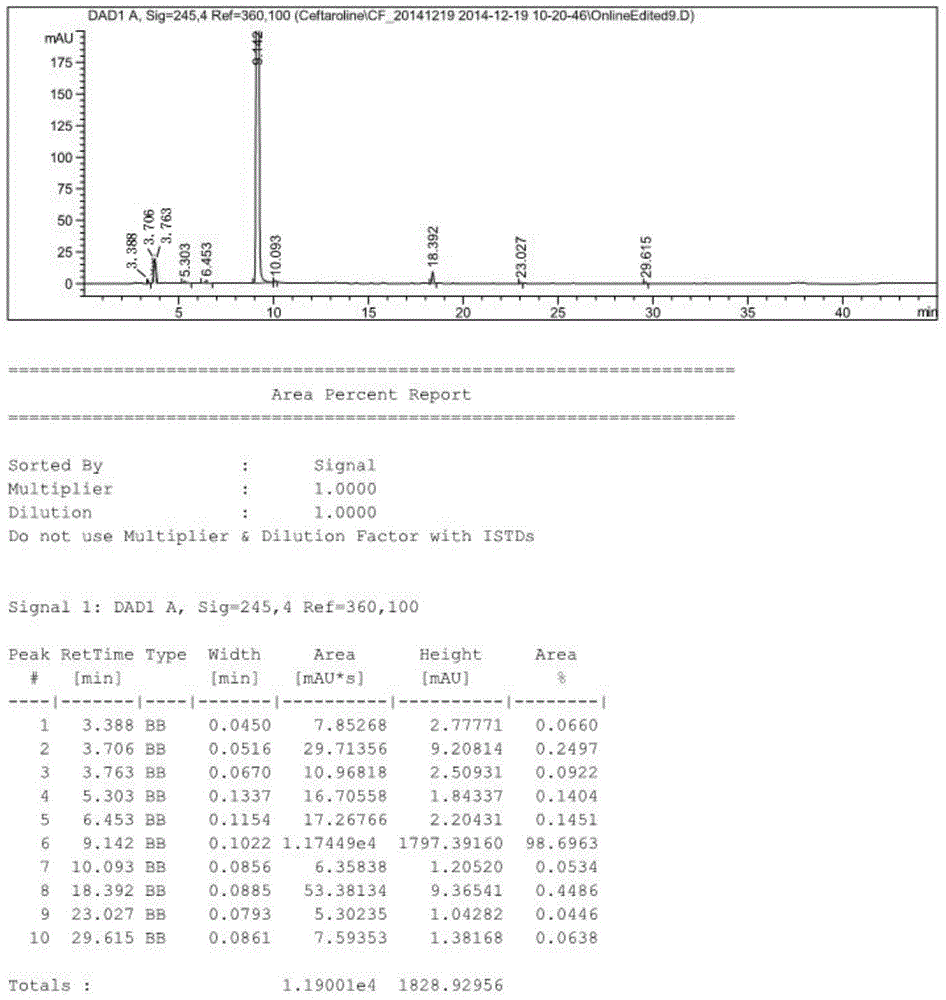
[0055] 1.1 1000g (2mol) 3-hydroxy cephalosporin, 304g (2.2mol) K 2 CO 3 Add 4L DMF to a four-neck flask, stir at room temperature for 0.5h, then cool in an ice-salt bath to -8°C to -10°C, slowly add 420g (2.42mol) p-toluenesulfonyl chloride (TosCl) dropwise, the reaction releases heat, control The dropping speed keeps the temperature of the reaction system not higher than -5°C. After the dropwise addition is completed, keep the reaction temperature in the ice-salt bath at -5°C~-8°C, and stir for 2 hours. After the raw materials are completely reacted by TLC, pour the reaction solution slowly. Pour into 40L of ice water, a large amount of solids precipitated, stirred at room temperature for 1 hour, then suction filtered, the filter cake was washed once with water, washed once with n-heptane compacted with water, dried in a blast drying oven at 60°C for 8-9 hours, and 1.3kg of off-white was obtained. Solid, the activated intermediate 7β-phenylacetamido-3-(4-methylbenzenesulfony...
Embodiment 2
[0065] 2.1 Mix 10kg (20mol) 3-hydroxycephalosporin, 3kg (22mol) K 2 CO 3Add 40L DMF to the reaction kettle, stir at room temperature for 1 hour, then cool in an ice-salt bath to -8°C to -10°C, slowly add 4.2kg (24.2mol) p-toluenesulfonyl chloride (TosCl) dropwise, the reaction releases heat, and the dropwise Make the temperature of the reaction system not higher than -5°C. After the dropwise addition, keep the reaction temperature in the ice-salt bath at -5°C~-8°C, and stir for 2 hours. After the raw materials are completely reacted by TLC, slowly pour the reaction solution into In 400L of ice water, a large amount of solids were precipitated, stirred at room temperature for 1 hour, then suction filtered, the filter cake was washed once with water, and dried in a vacuum oven at 40°C until the water content was less than 1%, and 13kg of off-white solid was obtained, which was the activated intermediate 7β-phenylacetyl Diphenylmethyl amino-3-(4-methylbenzenesulfonyloxy)-3-cephe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com