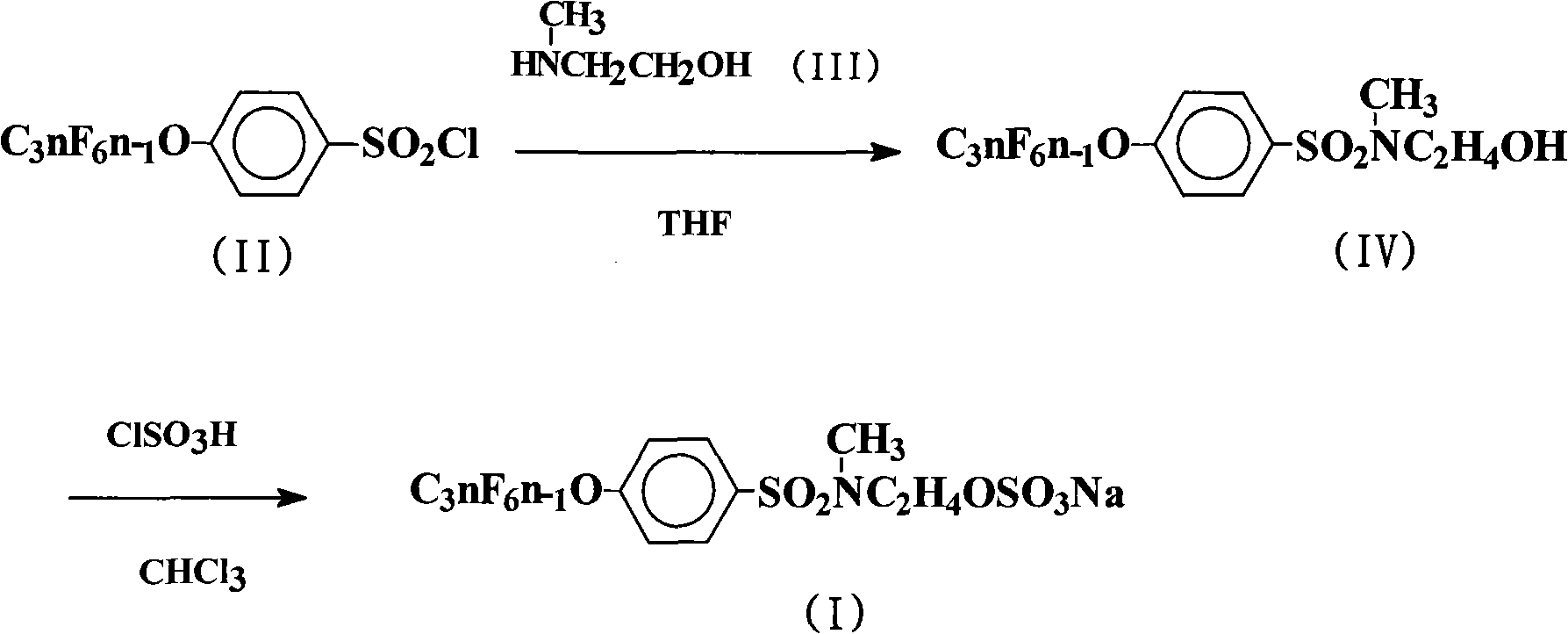
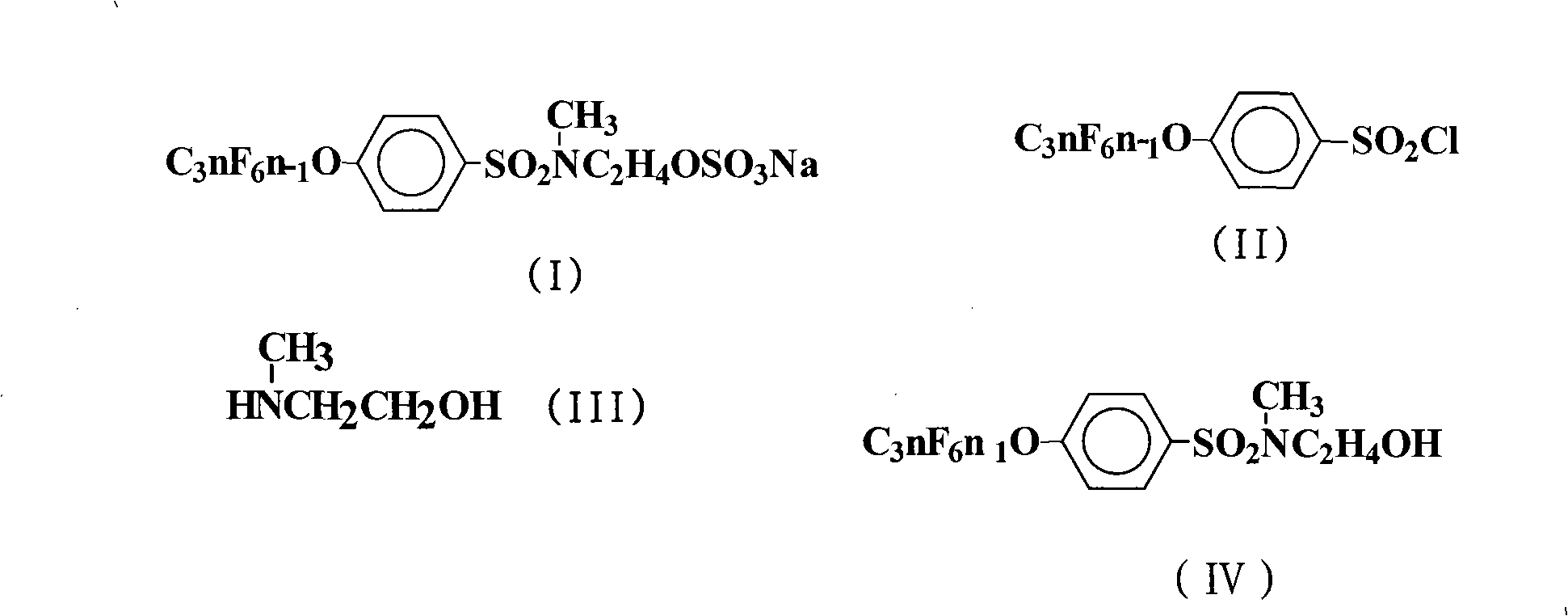Method for producing sulfuric acid fluorinated surfactants
A technology of fluorosurfactant and sulfate ester salt is applied in the field of preparation of sodium 2-ethyl sulfate, and achieves the effects of less waste, high reaction yield and low surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1N-hydroxyethyl-N-methyl-4-perfluoroalkenyloxybenzenesulfonamide
[0023]In a 500mL four-neck flask, add 225mL chlorobenzene, 124.5g (0.2mol) 4-perfluorononenyloxybenzenesulfonyl chloride (Lianyungang Taizhuo New Material Co., Ltd.), 15.0g (0.2mol) 2-methylaminoethanol , 21.2g (0.2mol) of anhydrous sodium carbonate, stirred, heated to 100°C, and kept stirring at the temperature for 12h. The reaction solution was distilled under reduced pressure until no solvent came out. Cool to room temperature, wash the obtained viscous oil, let it stand, and recrystallize with chlorobenzene to obtain 122.6 g of white crystal N-hydroxyethyl-N-methyl-4-perfluorononenyloxybenzenesulfonamide, the yield 92.7%.
Embodiment 2
[0024] The preparation of embodiment 2N-hydroxyethyl-N-methyl-4-perfluoroalkenyloxybenzenesulfonamide
[0025] React according to the method of Example 1, but the 2-methylaminoethanol feed intake is 75.1g (1.0mol), 21.2g (0.1mol) of anhydrous sodium carbonate is changed to 24g (0.6mol) sodium hydroxide, and the amidation reaction temperature is changed to 120°C, keep the temperature and stir for 24h. The reaction solution was distilled under reduced pressure until no solvent came out. Cool to room temperature, wash the obtained viscous oil, let it stand, and recrystallize with chlorobenzene to obtain 124.7.0 g of white crystal N-hydroxyethyl-N-methyl-4-perfluorononenyloxybenzenesulfonamide. The rate is 94.3%.
Embodiment 3
[0026] The preparation of embodiment 3N-hydroxyethyl-N-methyl-4-perfluoroalkenyloxybenzenesulfonamide
[0027] Carry out reaction according to embodiment 1 method, but solvent is changed into 270mL trichloroethane, 2-methylaminoethanol feed intake is 24.0g (0.32mol), 21.2g (0.1mol) anhydrous sodium carbonate is changed into 40.5g (0.4mol) Triethylamine, the amidation reaction temperature was changed to 80°C, and the temperature was kept stirring for 16h. The reaction solution was distilled under reduced pressure until no solvent came out. Cool to room temperature, wash the obtained viscous oil, let it stand, and recrystallize with chlorobenzene to obtain 123.0 g of white crystal N-hydroxyethyl-N-methyl-4-perfluorononenyloxybenzenesulfonamide, the yield 93.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com