Benzenesulphonyl fluoride, preparing method and application thereof
A technology for benzenesulfonyl fluoride compounds, which is applied to benzenesulfonyl fluoride compounds and the fields of preparation and application thereof, can solve the problems of loss of stereochemical information, insufficient detection sensitivity, erroneous detection results, etc., and achieves stable and stable derivatized products. High instrument response value and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
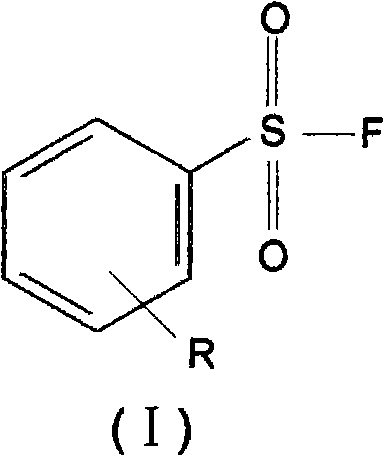
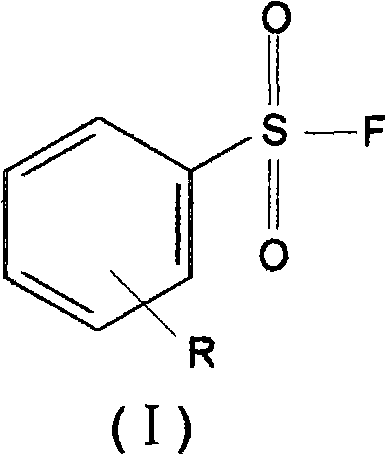
[0023] 4-Methoxybenzenesulfonyl fluoride CH 3 O-C 6 h 4 -SO 2 Synthesis of F:
[0024] Add 10.5 g of sodium fluoride, 4008 g of polyethylene glycol and 200 ml of acetonitrile into a 500 ml four-neck round bottom flask with a stirrer, a reflux condenser, a thermometer jack and a feed port. Stir on a 30°C water bath for 60 minutes until sodium fluoride particles of uniform size are observed. Then, a suspension of 200 ml of acetonitrile containing 20.6 g of 4-methoxybenzenesulfonyl chloride was added to the above-mentioned four-neck flask.
[0025] Stirring was continued at 30°C for 120 minutes before the reaction was stopped. Pour the fluorinated reactant suspension into a 1000ml beaker filled with 400ml of distilled water under stirring, and let it stand overnight.
[0026] The fluorinated product was filtered out on a Buchner funnel, washed with ethanol to remove acetonitrile, dried and weighed to obtain 14 g of crude product of 4-methoxybenzenesulfonyl fluoride. Recrys...
Embodiment 2
[0029] 2-Methoxy 4-methylbenzenesulfonyl fluoride 2-CH 3 O4-CH 3 -C 6 h 4 -SO 2 Synthesis of F:
[0030] Add 12.6 g of sodium fluoride, 6008 g of polyethylene glycol and 200 ml of acetonitrile into a 500 ml four-necked flask with a stirrer, a reflux condenser, a thermometer jack and an inlet. Stir on a 30°C water bath for 60 minutes until sodium fluoride particles of uniform size are observed. Then, a suspension of 200 ml of acetonitrile containing 22 g of 2-methoxyl 4-methylbenzenesulfonyl chloride was added to the above-mentioned four-neck flask.
[0031] Stirring was continued at 30°C for 120 minutes before the reaction was stopped. Pour the fluorinated reactant suspension into a 1000ml beaker filled with 400ml of distilled water under stirring, and let it stand overnight.
[0032] The fluorinated product was filtered out on a Buchner funnel, washed with ethanol to remove acetonitrile, dried and weighed to obtain 14.2 g of crude 2-methoxy 4-methylbenzenesulfonyl fluo...
Embodiment 3
[0046] Determination of secondary amino acids: proline, hydroxyproline, and N-ethylglycine using 4-methoxybenzenesulfonyl fluoride as a labeling reagent:
[0047] Instruments: High performance liquid chromatography from Waters, USA: 510 pump, U6k injector, 490E ultraviolet detector, reversed-phase C8 chromatographic column.
[0048] Reagents: proline, hydroxyproline, N-ethylglycine (Aldrich product);
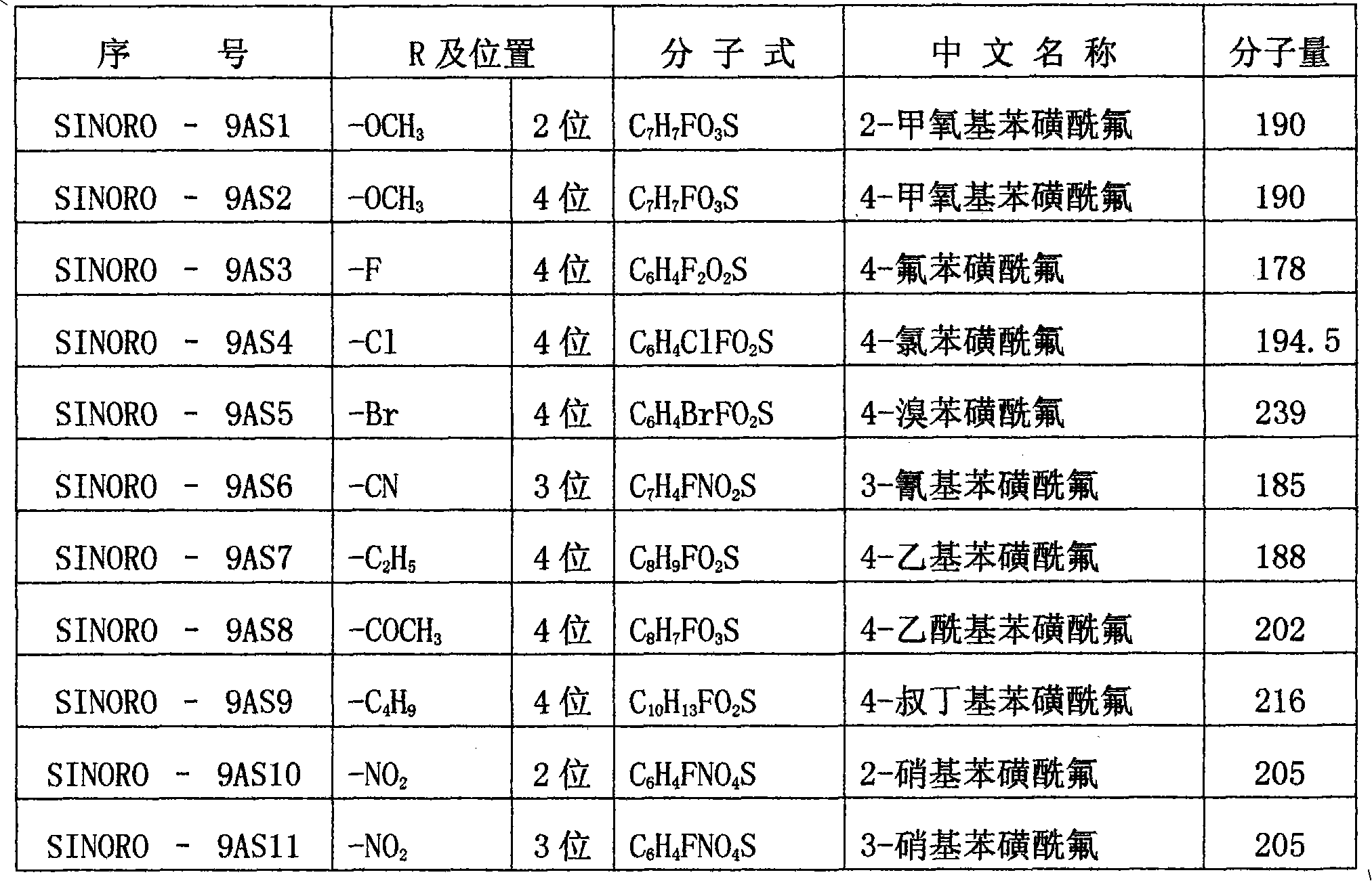
[0049] 4-methoxybenzenesulfonyl fluoride (SINORO-9AS2, self-made) in the series of benzenesulfonyl fluoride compounds;
[0050] Mobile phase: Accurately weigh 6.8g of crystalline sodium acetate, add about 850ml of deionized aqueous solution, adjust the pH to 4.2 with 10% acetic acid solution, add deionized water to 1000ml, filter with suction, take 770ml of this solution, add 230ml of chromatographically pure acetonitrile and mix well , degassed for standby;
[0051] Methods: ① Accurately prepare 100 μl each of proline aqueous solution, hydroxyproline aqueous solution, N-ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com