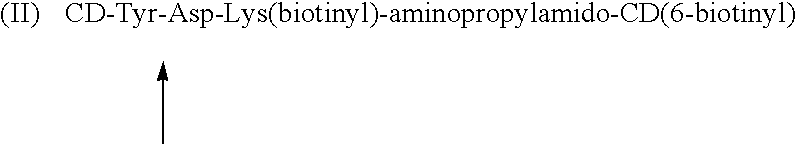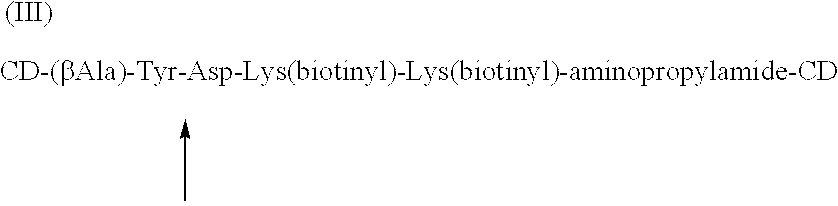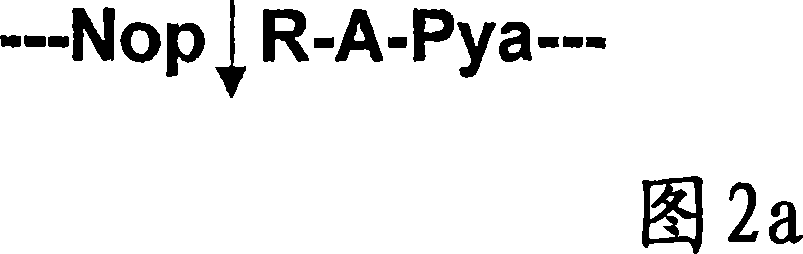Patents
Literature
99 results about "Peptide structure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
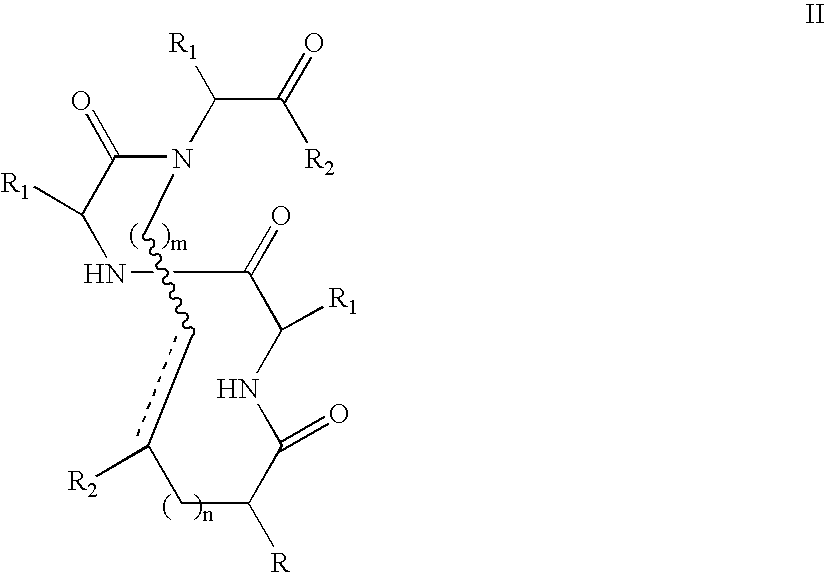
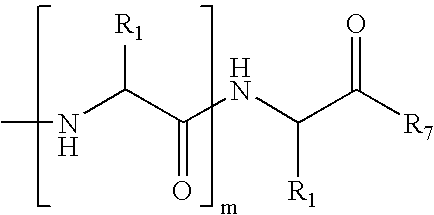
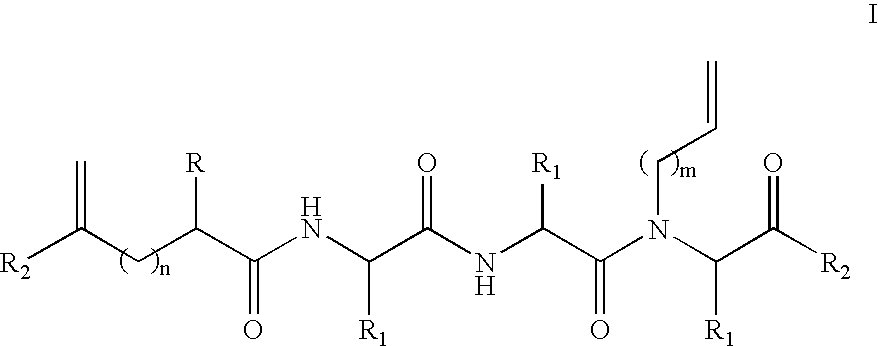
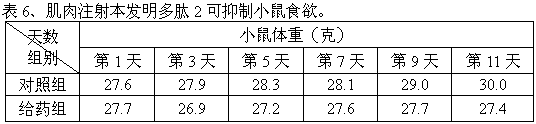
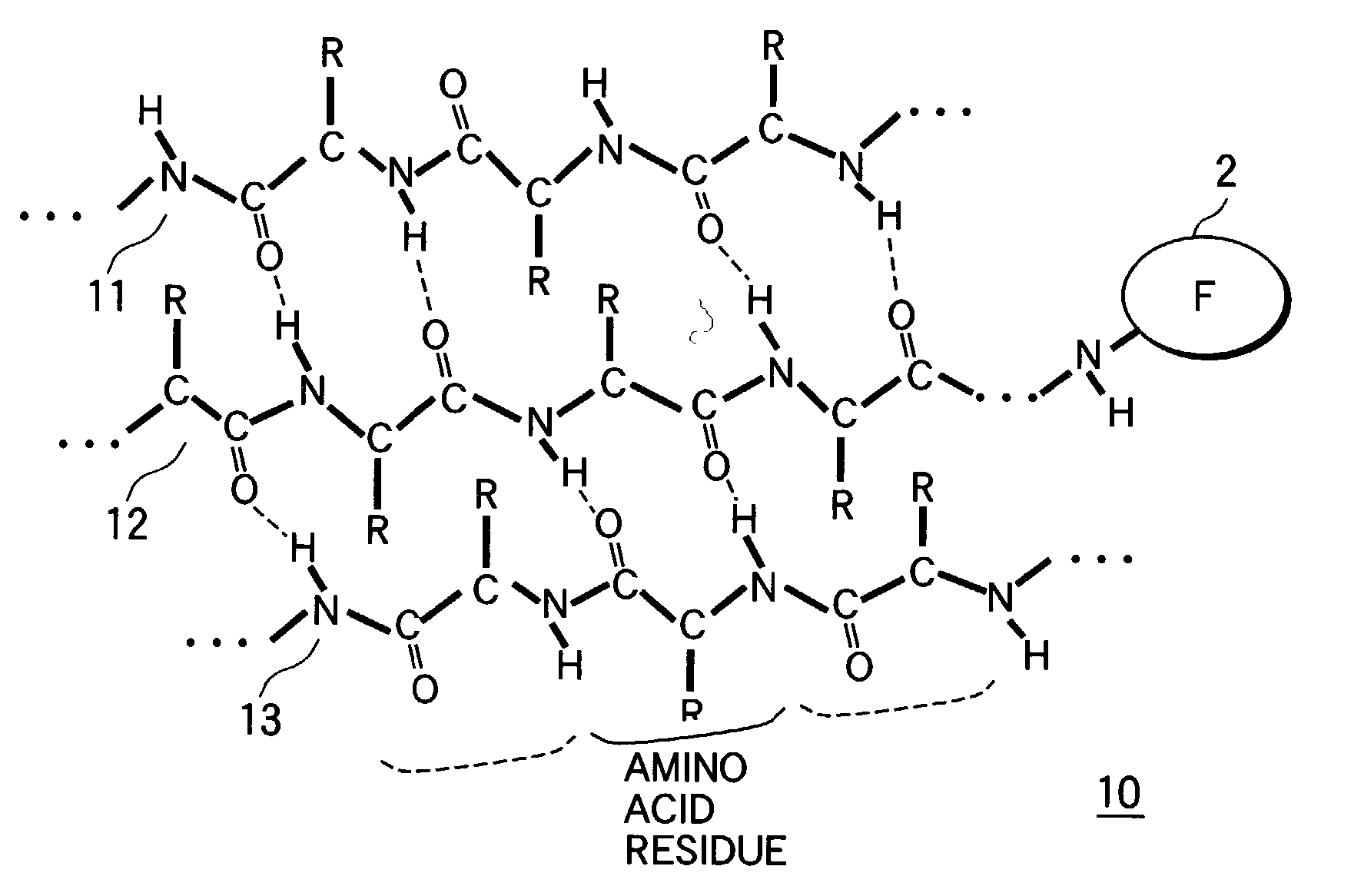
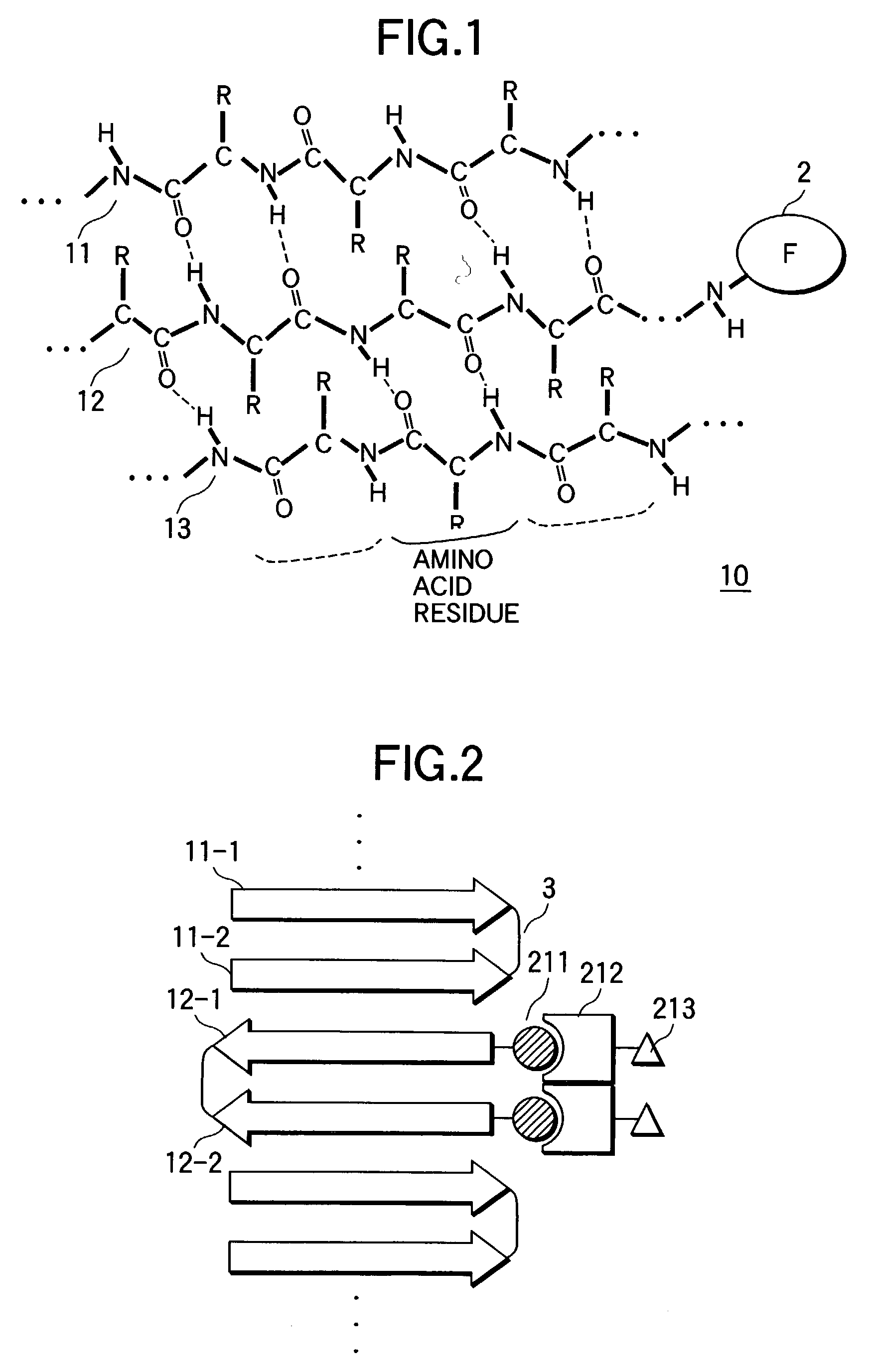
The peptide molecule is a linear or branched chain. If the molecule is linear, it has two termini with one terminal amino group (—NH2) and one terminal carboxyl group (—COOH). Peptides with a closed-chain structure are called cyclopeptides, which include many bacterial toxins, hormones, and antibiotics.
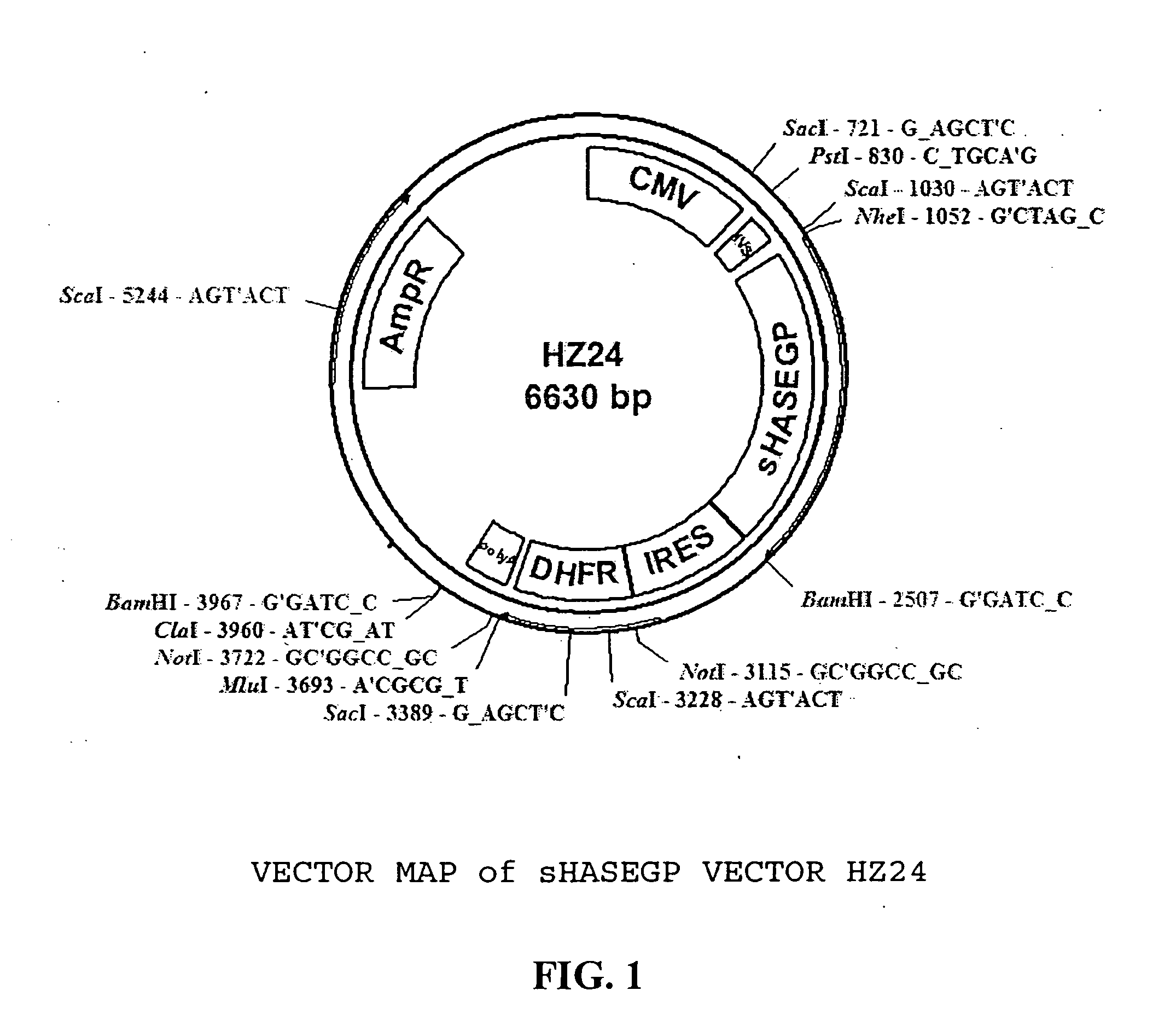
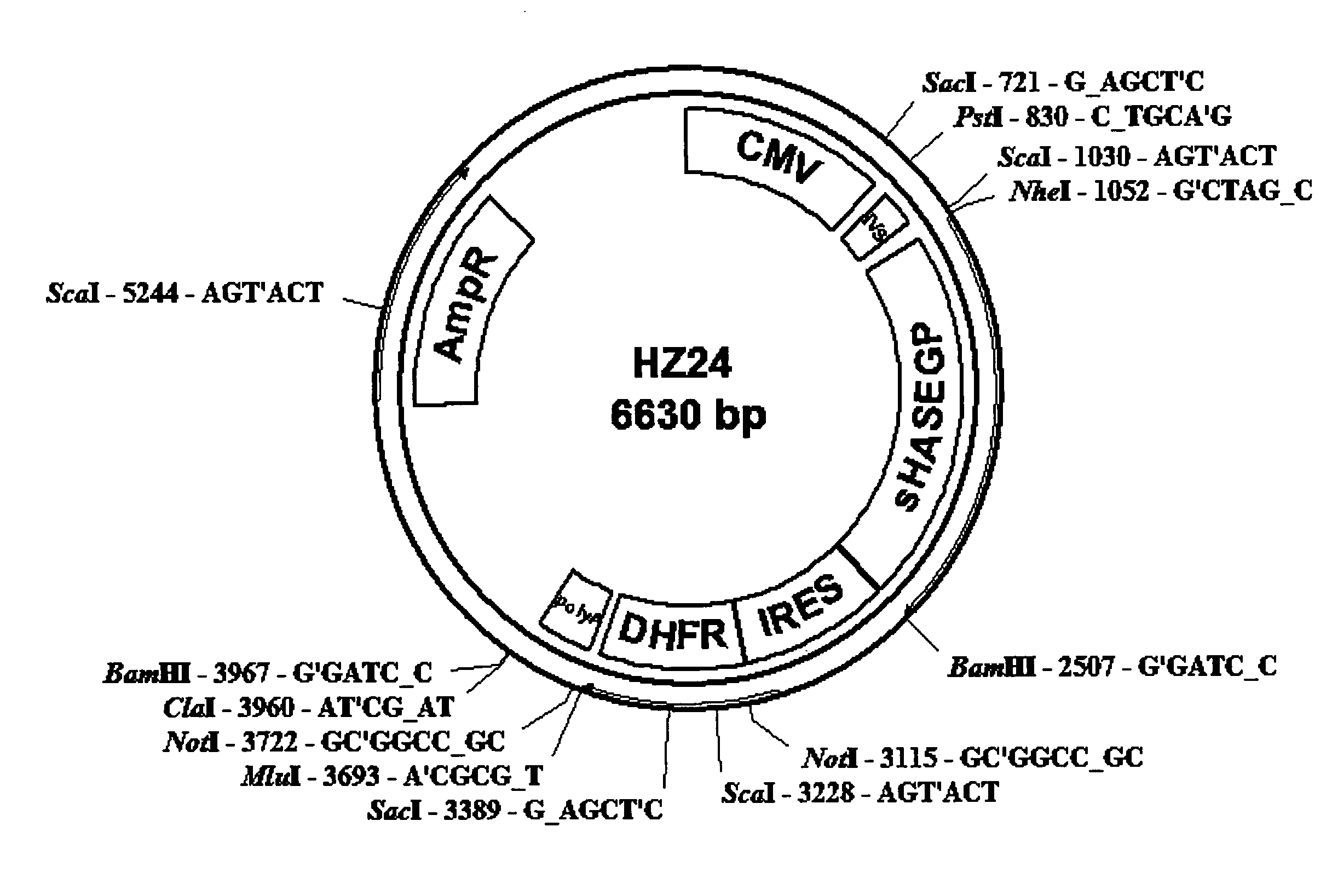
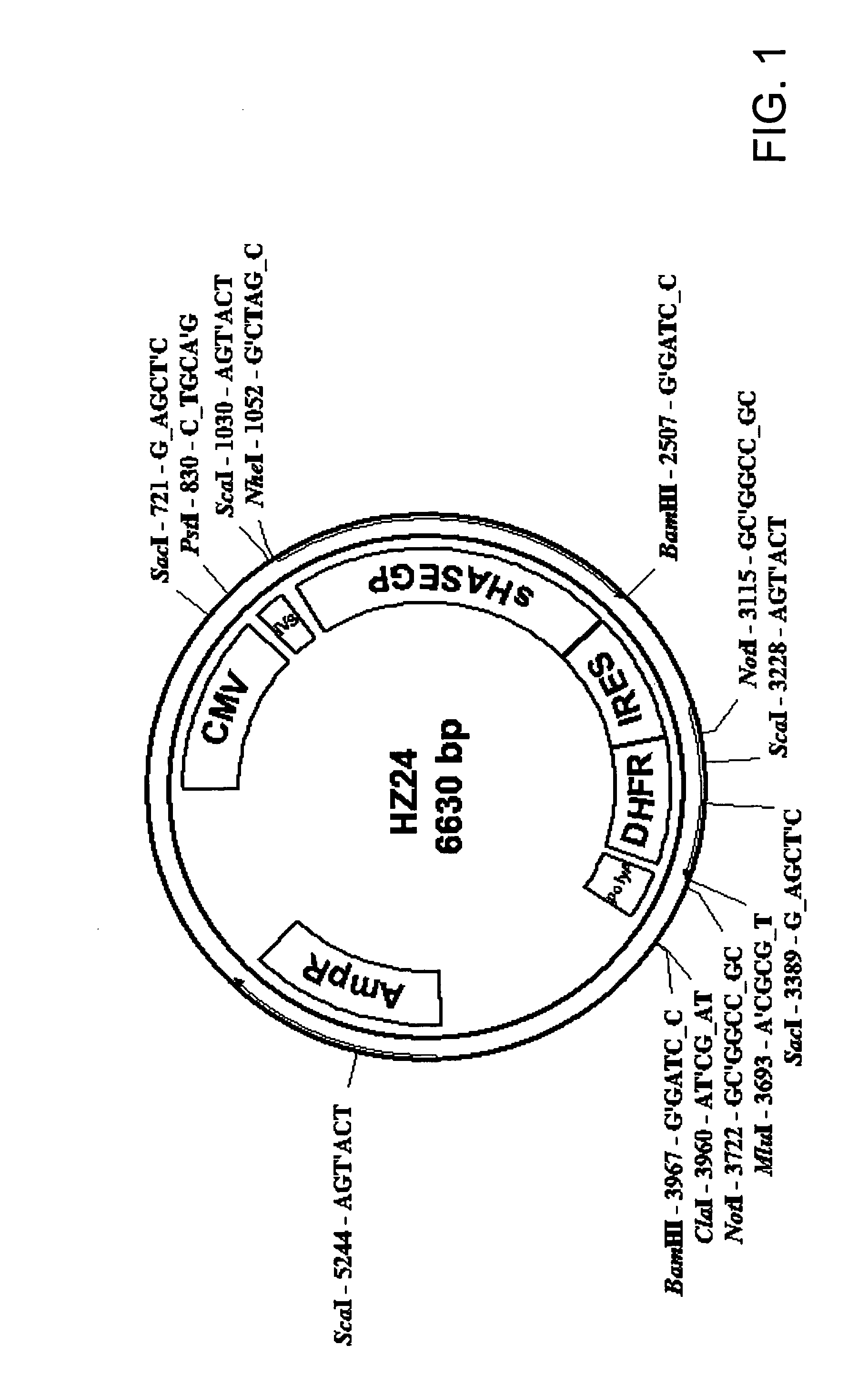
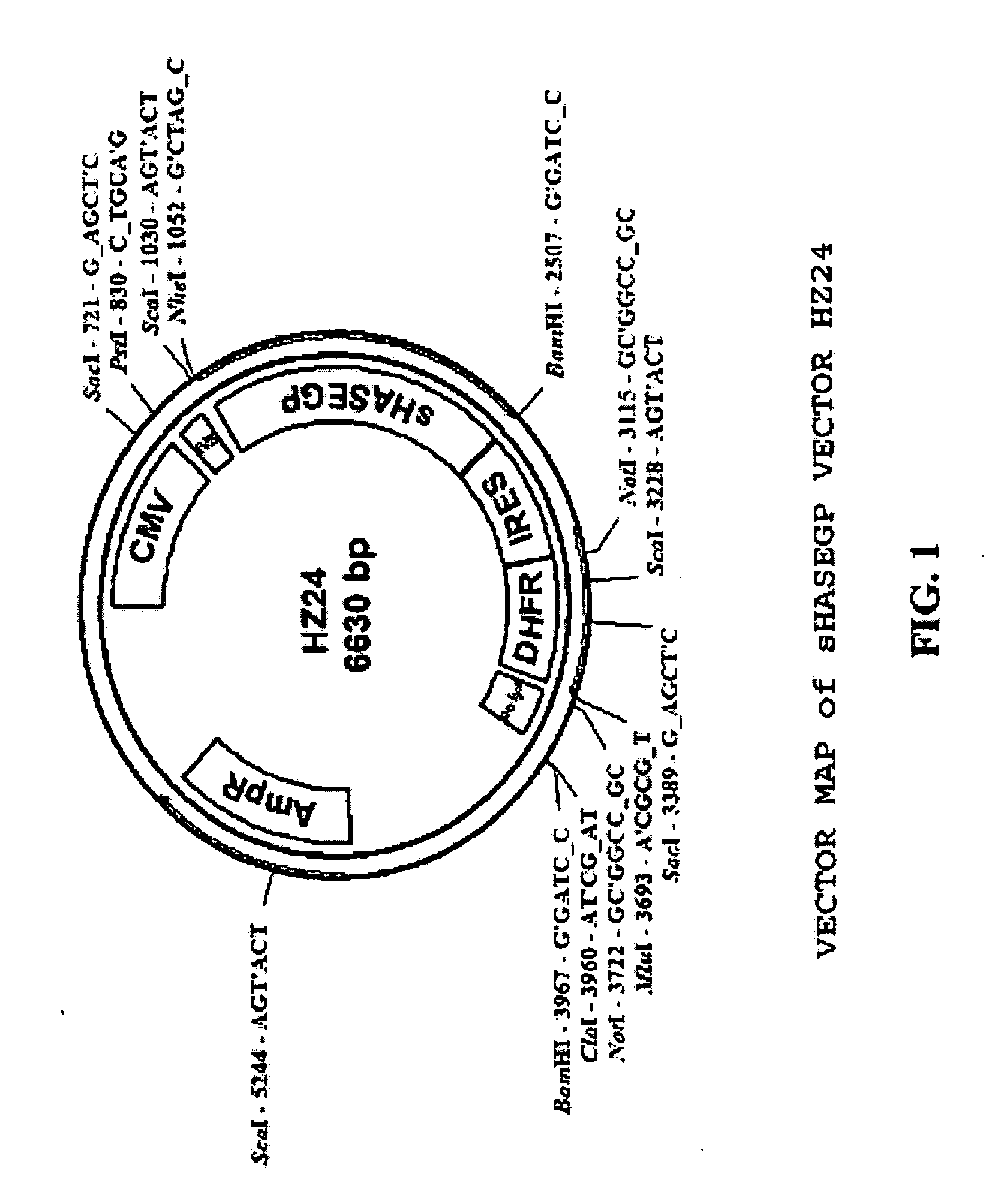
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
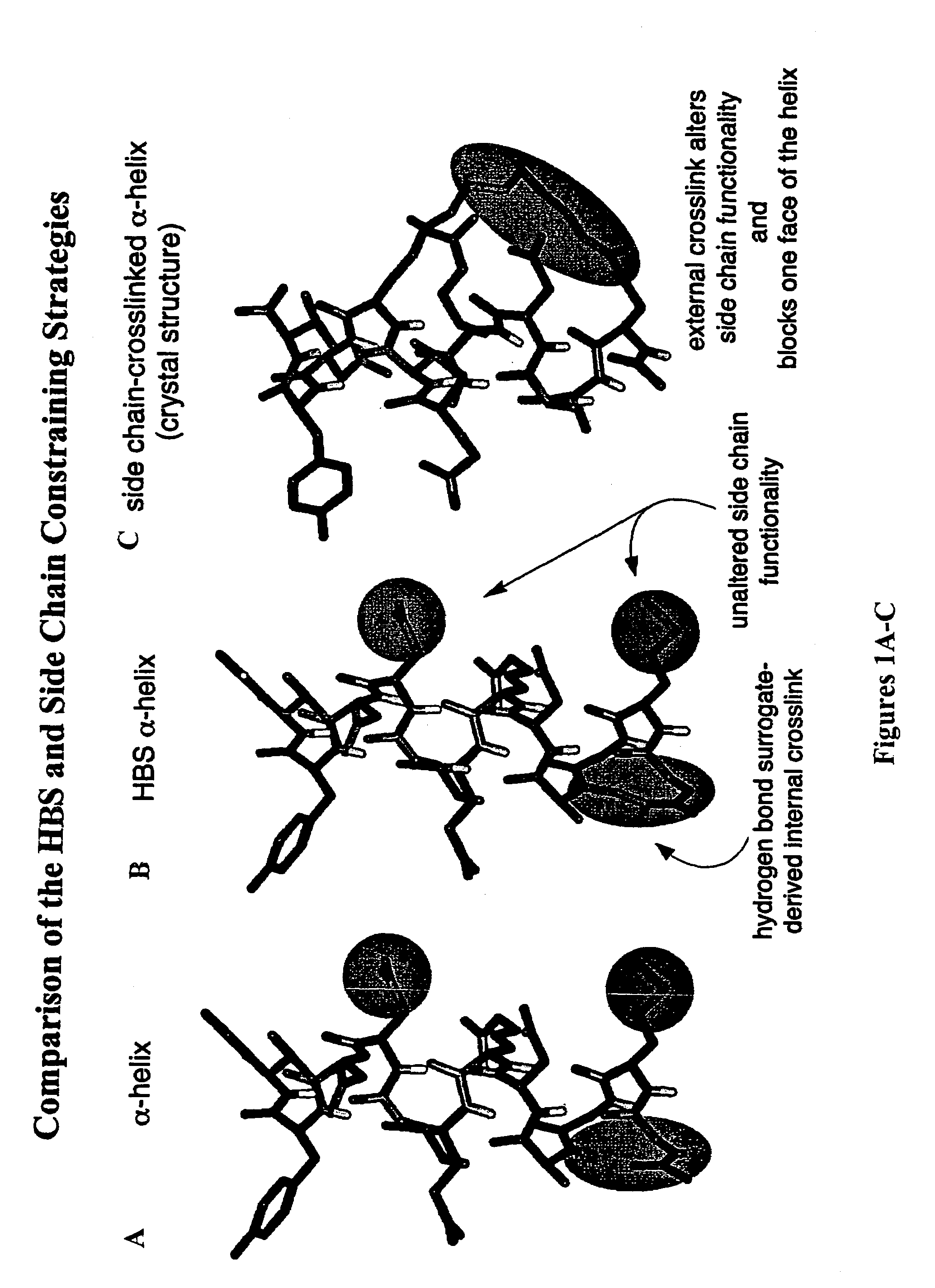
Methods for preparing internally constrained peptides and peptidomimetics
ActiveUS7202332B2Stable and irreversible bondPeptide preparation methodsCyclic peptide ingredientsBeta sheetHelix
The present invention relates to a method for preparing a peptide having a stable, internally constrained alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region and a method of stabilizing an alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region within a peptide structure. The resulting peptides and methods of using them are also disclosed.
Owner:NEW YORK UNIV
Methods for preparing internally constrained peptides and peptidomimetics
ActiveUS20060014675A1Promote cell deathAvoid interactionPeptide preparation methodsImmunoglobulinsGreek letter betaBeta sheet
The present invention relates to a method for preparing a peptide having a stable, internally constrained alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region and a method of stabilizing an alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region within a peptide structure. The resulting peptides and methods of using them are also disclosed.
Owner:NEW YORK UNIV
Intermedin analogue prepared by bonding ring core sequence with biotin or cell-penetrating peptides
The invention relates to an intermedin analogue prepared by bonding a ring core sequence with biotin or cell-penetrating peptides and a preparation method and application of the intermedin analogue. The intermedin analogue is prepared by bonding a functional unit with the biotin or a load unit; the functional unit is a seven-peptide structure formed by connecting the ring core sequence c (Asp-Dab-D-Phe-Arg-Trp-Lys) with a connection unit Arg, the structure is Arg-c (Asp-Dab-D-Phe-Arg-Trp-Lys); the load unit is the 48th-57th peptide segments Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg or the 55th-57th peptide segments Arg-Arg-Arg in the 47th-60th segments of HIV (human immunodeficiency virus)-1TAT protein. The intermedin analogue disclosed by the invention can penetrate through mucosa or skin to be absorbed, and can be applied to treatment of diseases such as male and female sexual dysfunction, obesity, pigmentation deficiency and the like.
Owner:张嘎
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090181013A1Reduce deliveryImprove usabilityAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20100196423A1Reduce sensitivityGreater serum half-livesAntibacterial agentsSenses disorderHyaluronidaseNuclear chemistry
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated form of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Tumor cell targeting penetrating peptide
InactiveCN104140457ARemarkable penetration effectAntibody mimetics/scaffoldsDepsipeptidesDiseaseWilms' tumor
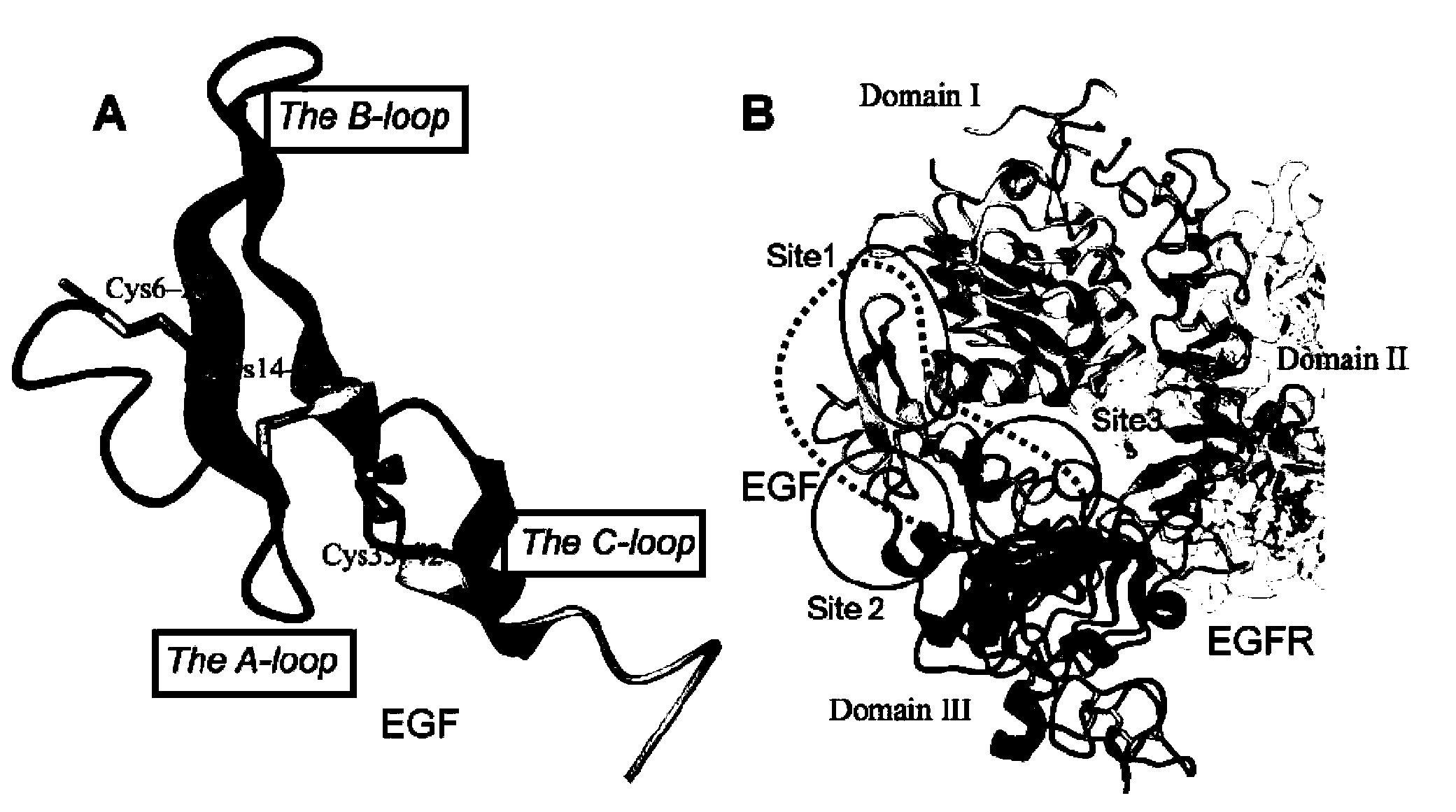
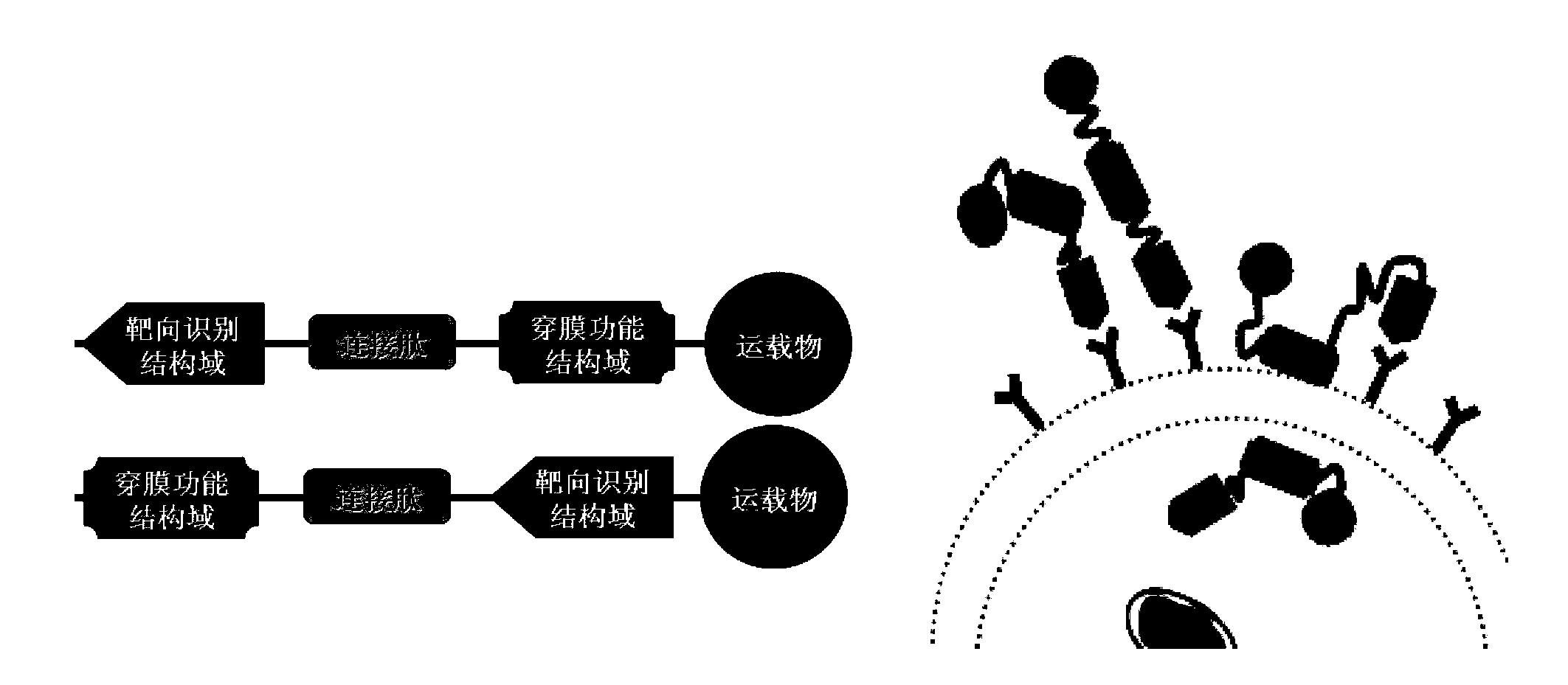
The invention relates to the biotechnical field, and concretely relates to a tumor cell targeting penetrating peptide, a polynucleotide molecule and a use thereof, and a targeting drug delivery system containing the tumor cell targeting penetrating peptide. The tumor cell targeting penetrating peptide is characterized in that the tumor cell targeting penetrating peptide contains a CPPs structure domain having cell penetrating peptide activity and an EFG third ring sample mimic peptide structure domain with specific targeting affinity to tumor cells. The tumor cell targeting penetrating peptide has a substantial cell penetrating effect, has the novel characteristics of cell specificity and species specificity, and can be used to diagnose tumor diseases and develop medicines required in the treatment process.
Owner:ZHEJIANG REACHALL PHARMA
Primer, primer set, and nucleic acid amplification method and mutation detection method using the same
ActiveUS20080227104A1Efficient detectionStrong fluorescenceMethine/polymethine dyesSugar derivativesBase JMutation detection
The present invention provides a primer that effectively can detect, for example, the double helix structure of a nucleic acid. The primer is a labeled nucleic acid containing at least one structure represented by the following formula (16),where B is an atomic group having a nucleobase skeleton, E is an atomic group having a deoxyribose skeleton, a ribose skeleton, or a structure derived from either one of them, or an atomic group having a peptide structure or a peptoid structure, and Z11 and Z12 are each an atomic group that exhibits fluorescence and are identical to or different from each other.
Owner:DNAFORM
Antioxidation active peptides and method for preparing same
InactiveCN101284872AGood scavenging effectInhibit peroxidation reactionMicroorganism based processesPeptide preparation methodsBacillus nattoIon exchange
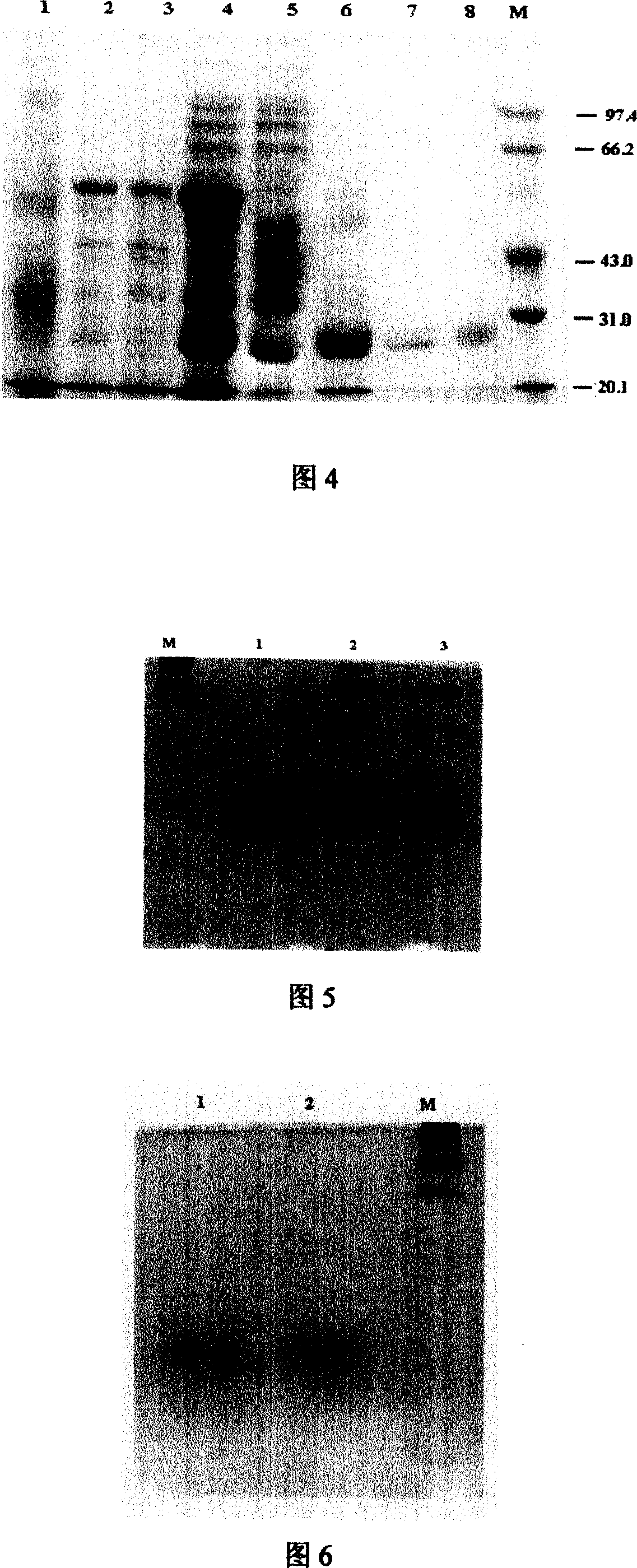
The invention discloses antioxidation bioactive peptide and a method for making the same. The product is made through the following steps that: microbial bacillus natto and corn protein powder are respectively used as strain and a raw material, which undergo liquid culture; and fermentation broth is separated through ion exchange chromatography, gel chromatography, reversed phase chromatogram and electrophoresis. The molecular weight of the product is between 20,100 and 31,000 and the five amino acid sequences at an N terminal are K-V-T-Y-H with the antioxidation activity reaching to 236.68U / mL. Moreover, the product has higher antioxidation activity, and pure product can meet the requirements of structural and physical property measurements of antioxidation bioactive peptide, activity and toxicological experiments as well as large-scale application such as therapy and cultivation; because antioxidation active material has strong cleaning action on superoxide anion free radical and hydroxyl free radical and can remarkably inhibit the overoxidation reaction between red blood cell and the lipid of liver, heart and brain tissue, the product has functions such as disease prevention and aging resistance and can be further developed into additives of products such as medicine and cosmetic.
Owner:QIQIHAR UNIVERSITY
Functional peptide fiber, production method thereof and method for recovering peptide chains
InactiveUS20030162696A1Easy to modifyFirmly connectedPeptide-nucleic acidsSynthetic resin layered productsFiberBeta sheet
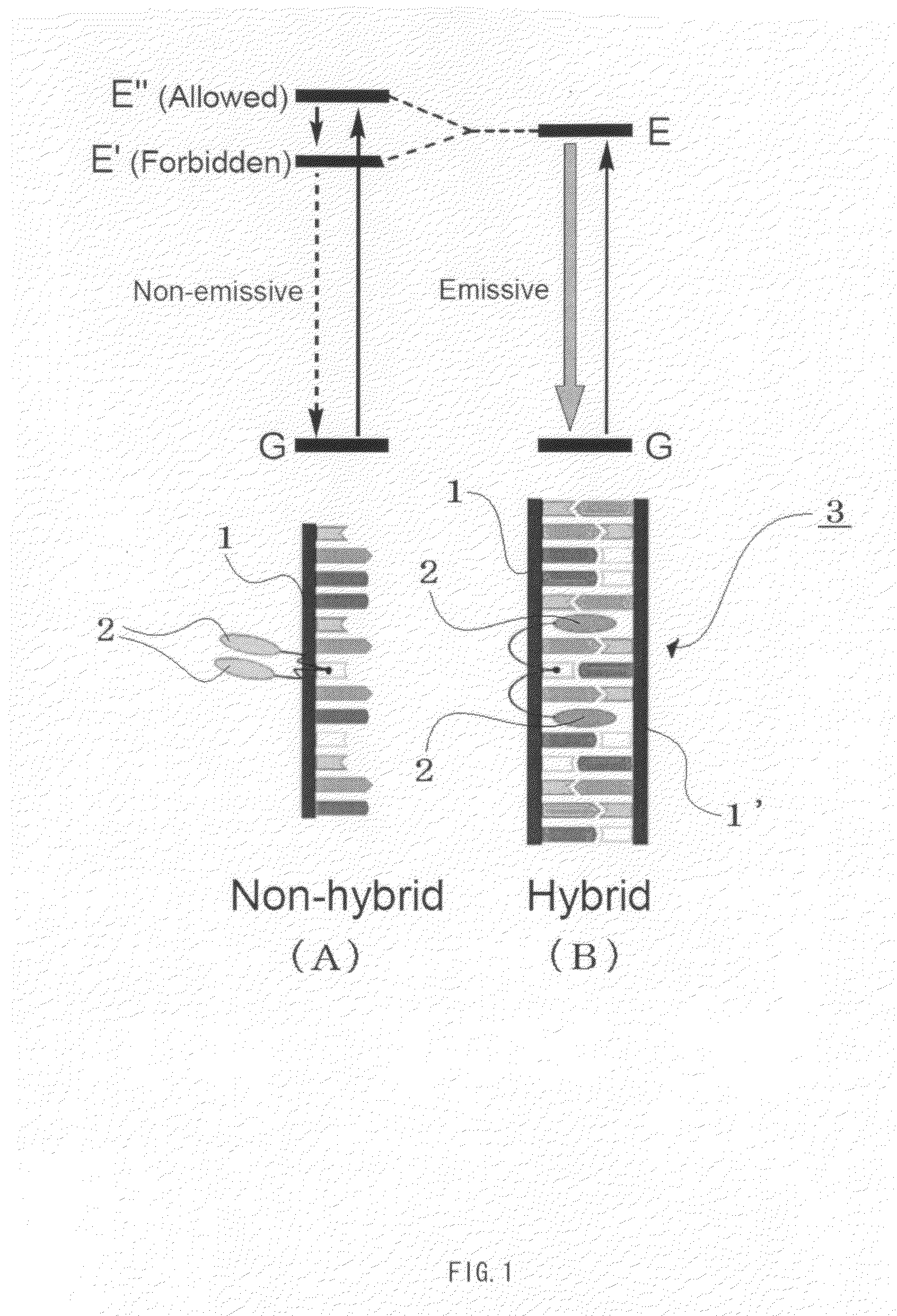
This invention provides a functional peptide fiber which comprises a plurality of peptide structure units each containing at least one peptide chain, wherein peptide chains contained in each adjacent peptide structure units do not form peptide bond but are structured into a fibrous form by taking a beta-sheet structure, and wherein at least one of the plurality of peptide structure units contains a peptide chain having a functional material connected thereto. Also disclosed are a method for producing the functional peptide fiber and a method for recovering peptide chains from the functional peptide fiber.
Owner:FUJIFILM BUSINESS INNOVATION CORP
Peptide-mediated gene transfer
InactiveUSRE39220E1Improve efficiencyProlong lifePeptide/protein ingredientsPeptide sourcesHinge regionPrimary cell
A methodology that allows for highly efficient transfer and stable integration of DNA into both established eukaryotic cell lines and primary cells, including non-dividing cells such as human peripheral blood monocytes and macrophages, entails the use of a synthetic polypeptide comprised of a peptide domain which corresponds to a nuclear localization signal sequence and a DNA binding domain which is rich in basic amino acids, separated by a hinge region of neutral acid which prevents stearic interference between the two domains.A synthetic polypeptide that allows for highly efficient transfer of DNA into eukaryotic cells, including, for example, non-dividing cells such as human peripheral blood monocytes and macrophages.<?insert-end id="INS-S-00001" ?>
Owner:GENETIC APPL
Beta-glucan peptide and its preparing process and application
InactiveCN1663959AImprove manufacturing speedHigh yieldPeptide/protein ingredientsDepsipeptidesChromatographic separationSide chain
A kind of beta-dextrantide, which is characterized in that the main chain is beta-1, 6-dextran and the side chain is beta-1, 3-dextran in dextrose chain. Defatting the gray-ramali cmycelium with ethanol, extracting with hot water and alkaline, neutralizing with acid, ultrafiltering, condensing and desalinizating, adding ethanol for deposition, separating by column chromatography, eluting using salinity gradient solvent, gathering and merging according to elution peak, ultrafiltering, condensing, desalinizating and drying. The invention can remove the mixed protein, heterosaccharide, and other impurity substance by separating for only one time and can get beta-dextrantide with high purity, simplifying the process and improving the preparing velocity and yield; and at the same time avoids the shortage of destructing the peptide structure when employing enzymatical method. The product can also be used for preparing immuno-modulatory agents and anti-cancer drugs.
Owner:OCEAN UNIV OF CHINA +1
Compound having structure derived from mononucleoside or mononucleotide, nucleic acid, labeling substance, and method and kit for detection of nucleic acid
ActiveUS20100092971A1Effective structureEfficient detectionSugar derivativesAntibioticsHydrogen atomFluorescence
The present invention provides, for example, a labeling substance that allows the double helix structure of a nucleic acid to be detected effectively. The present invention provides a compound having a structure derived from mononucleoside or mononucleotide, with the structure being represented by the following formula (1), (1b), or (1c), a tautomer or stereoisomer thereof, or a salt thereof.In the above formulae, B is an atomic group having a nucleobase skeleton, E is an atomic group having a deoxyribose skeleton, a ribose skeleton, or a structure derived from either one of them, or an atomic group having a peptide structure or a peptoid structure, and Z11 and Z12 each are a hydrogen atom, a protecting group, or an atomic group that exhibits fluorescence and may be identical to or different from each other.
Owner:RIKEN
Molecules and methods for inhibition and detection of proteins
The present application belongs to the field of functional peptides and more particularly to the field of controlled protein aggregation. The invention discloses molecules of a peptide structure as defined in the claims and methods of using such molecules for therapeutic applications and for diagnostic uses, as well as in other applications such as in the agbio field and in industrial biotechnology. The molecules can be used for curing and / or stabilizing infections such as bacterial,fungal and viral diseases, but are also useful in non-infectious human and veterinary diseases. The molecules can also be used for the detection of protein biomarkers and for the prognosis and diagnosis of a variety of diseases.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +3
Compound having structure derived from mononucleoside or mononucleotide, nucleic acid, labeling substance, and method and kit for detection of nucleic acid
The present invention provides, for example, a labeling substance that allows the double helix structure of a nucleic acid to be detected effectively. The present invention provides a compound having a structure derived from mononucleoside or mononucleotide, with the structure being represented by the following formula (1), (1b), or (1c), a tautomer or stereoisomer thereof, or a salt thereof.In the above formulae, B is an atomic group having a nucleobase skeleton, E is an atomic group having a deoxyribose skeleton, a ribose skeleton, or a structure derived from either one of them, or an atomic group having a peptide structure or a peptoid structure, and Z11 and Z12 each are a hydrogen atom, a protecting group, or an atomic group that exhibits fluorescence and may be identical to or different from each other.
Owner:RIKEN
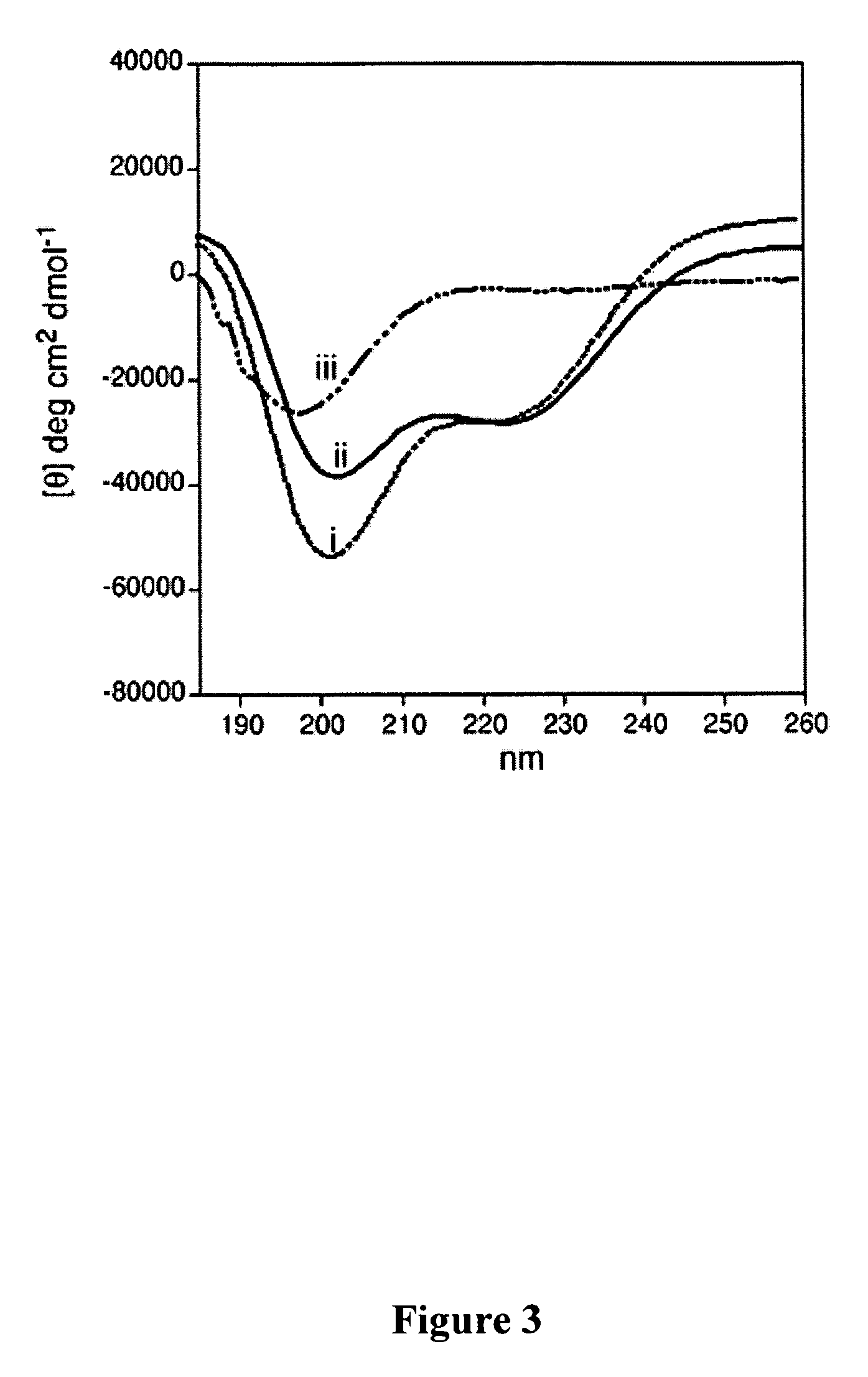
Synthetic approach to designed chemical structures
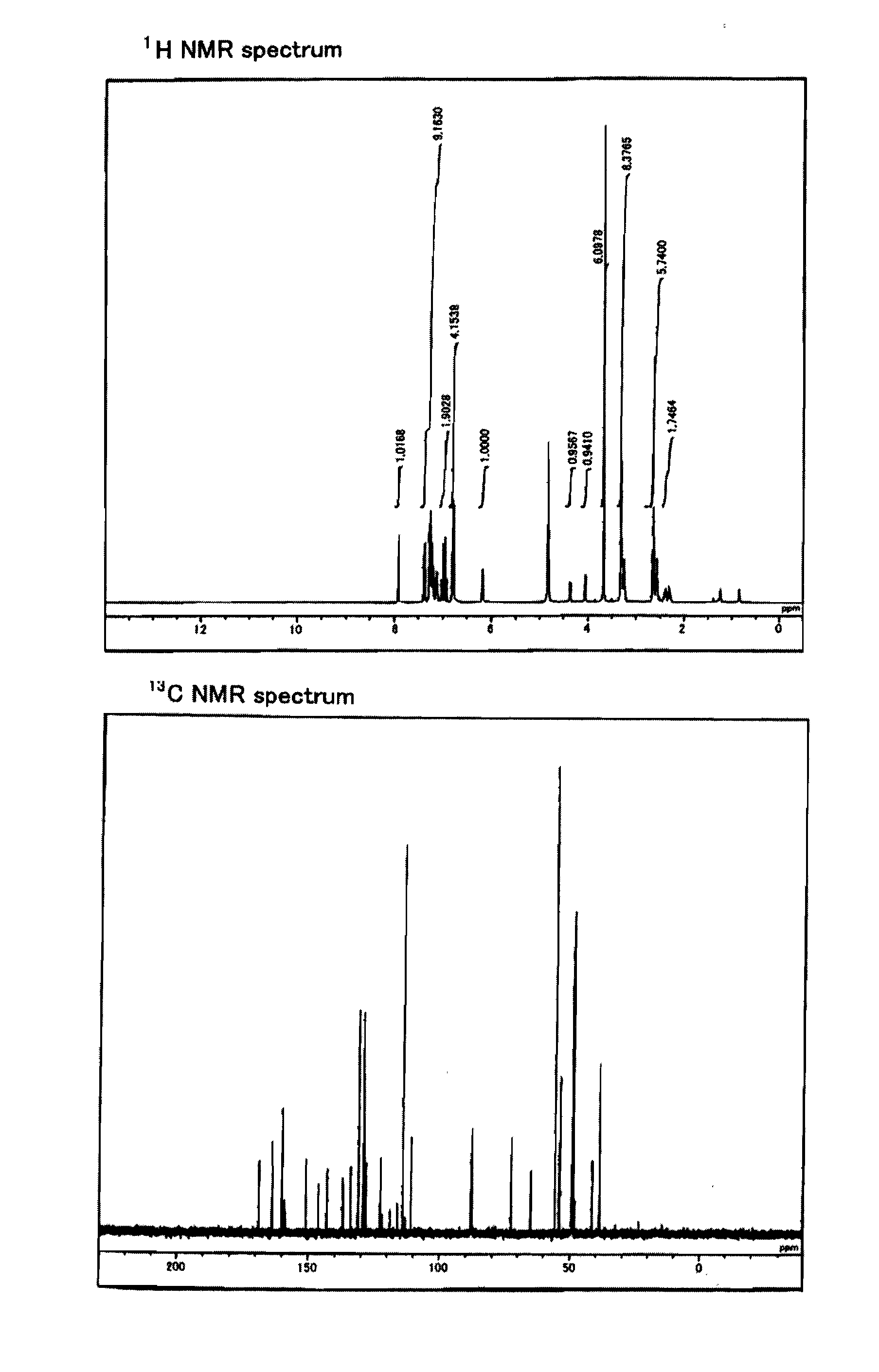
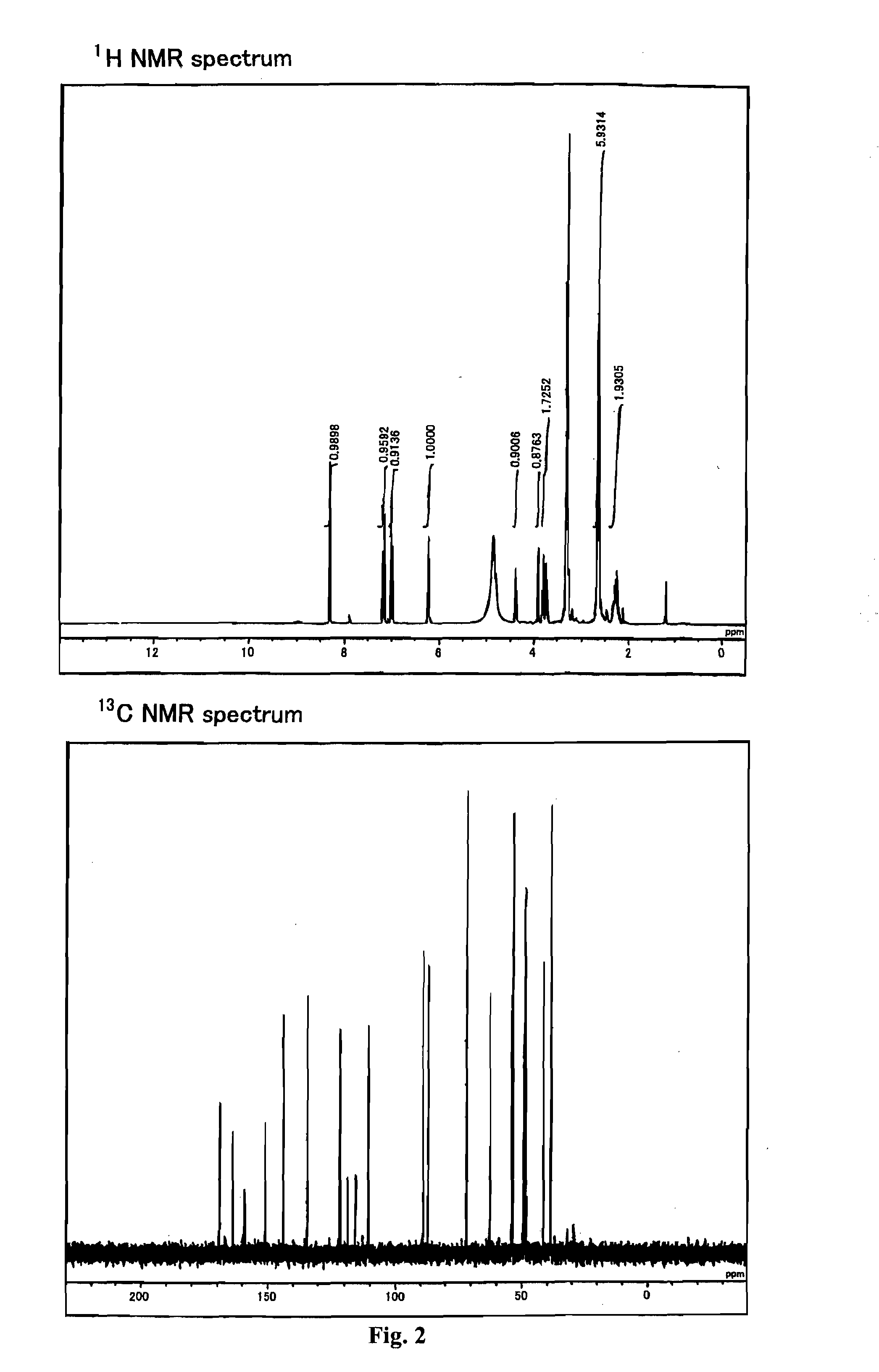
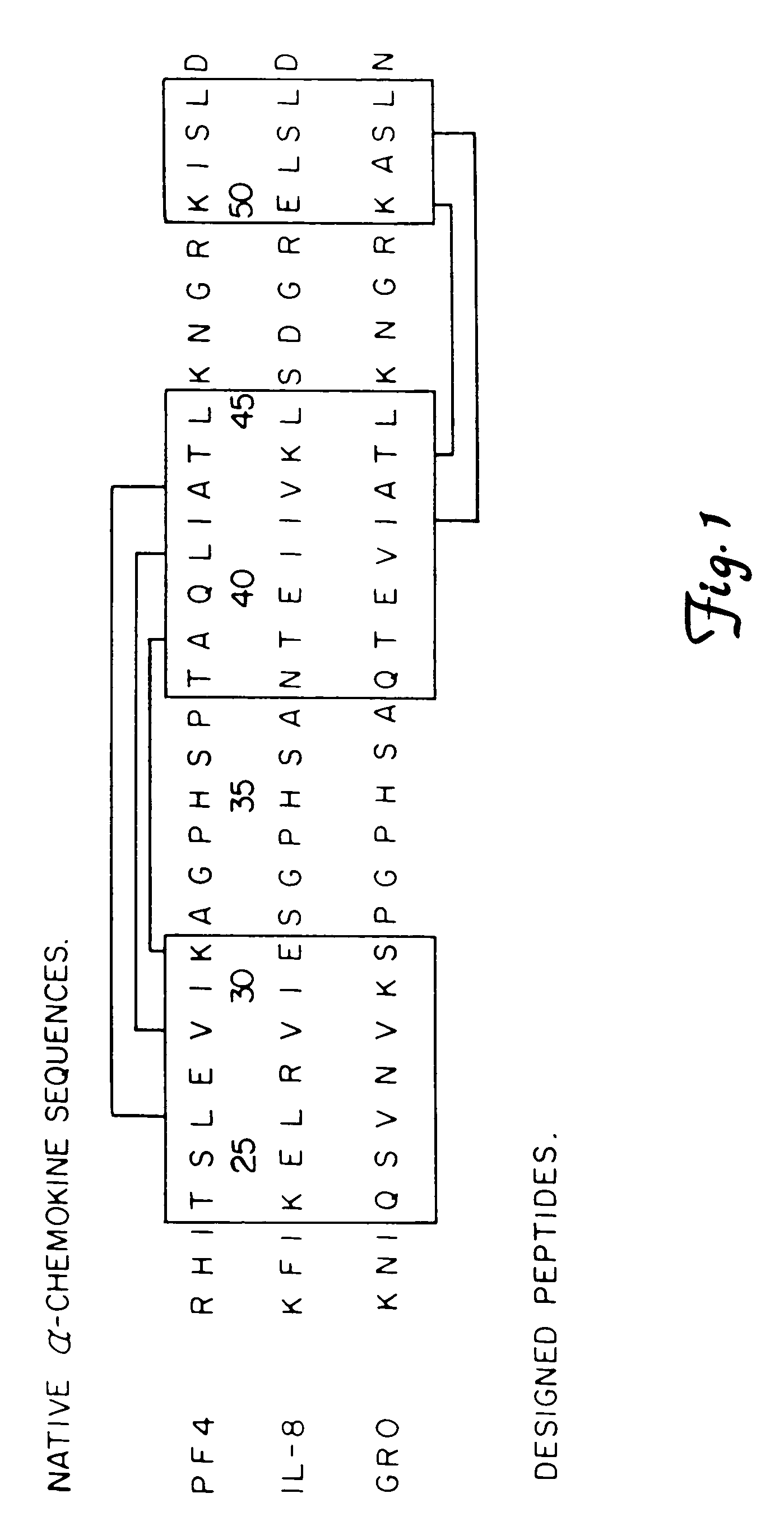
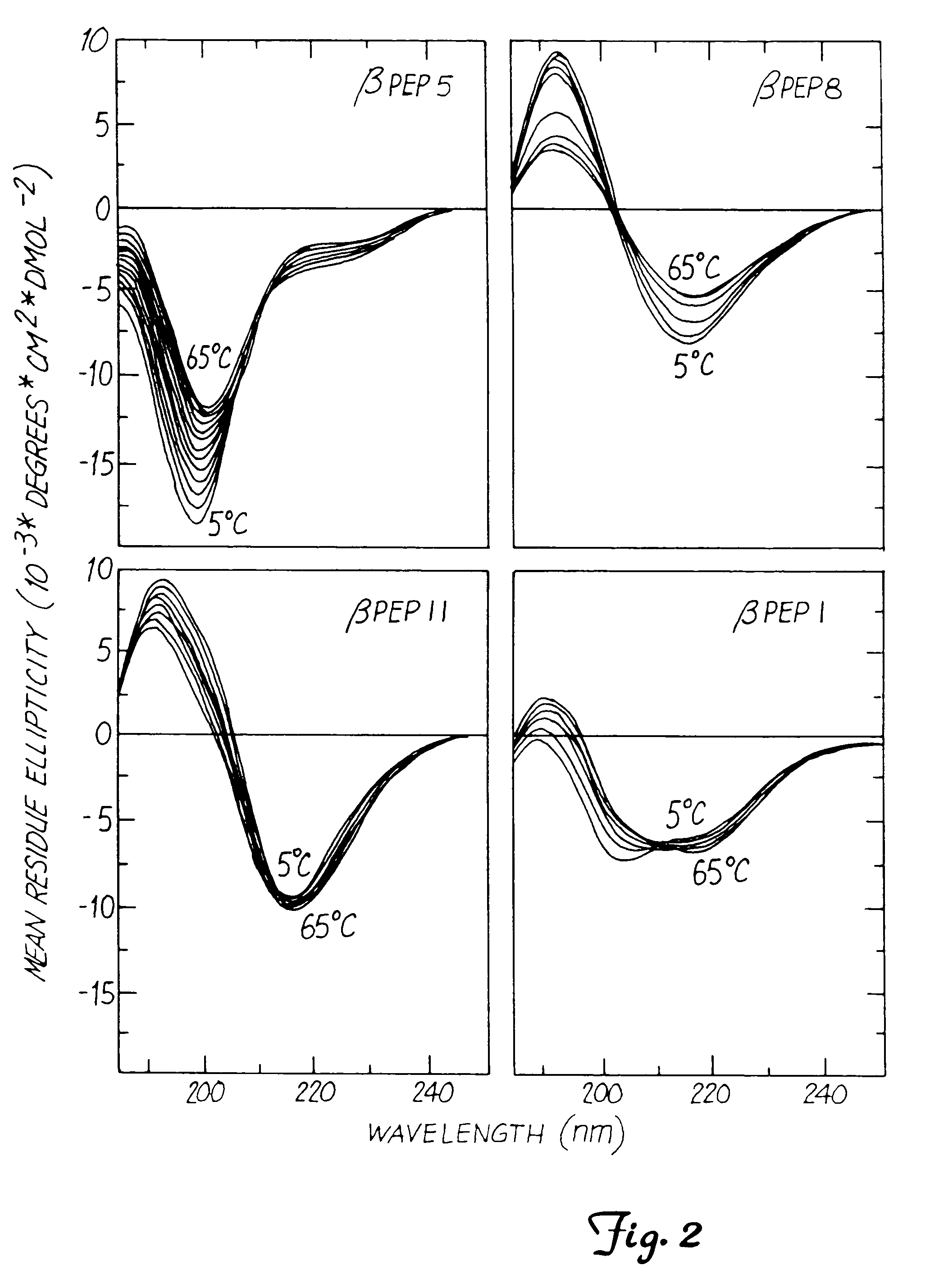
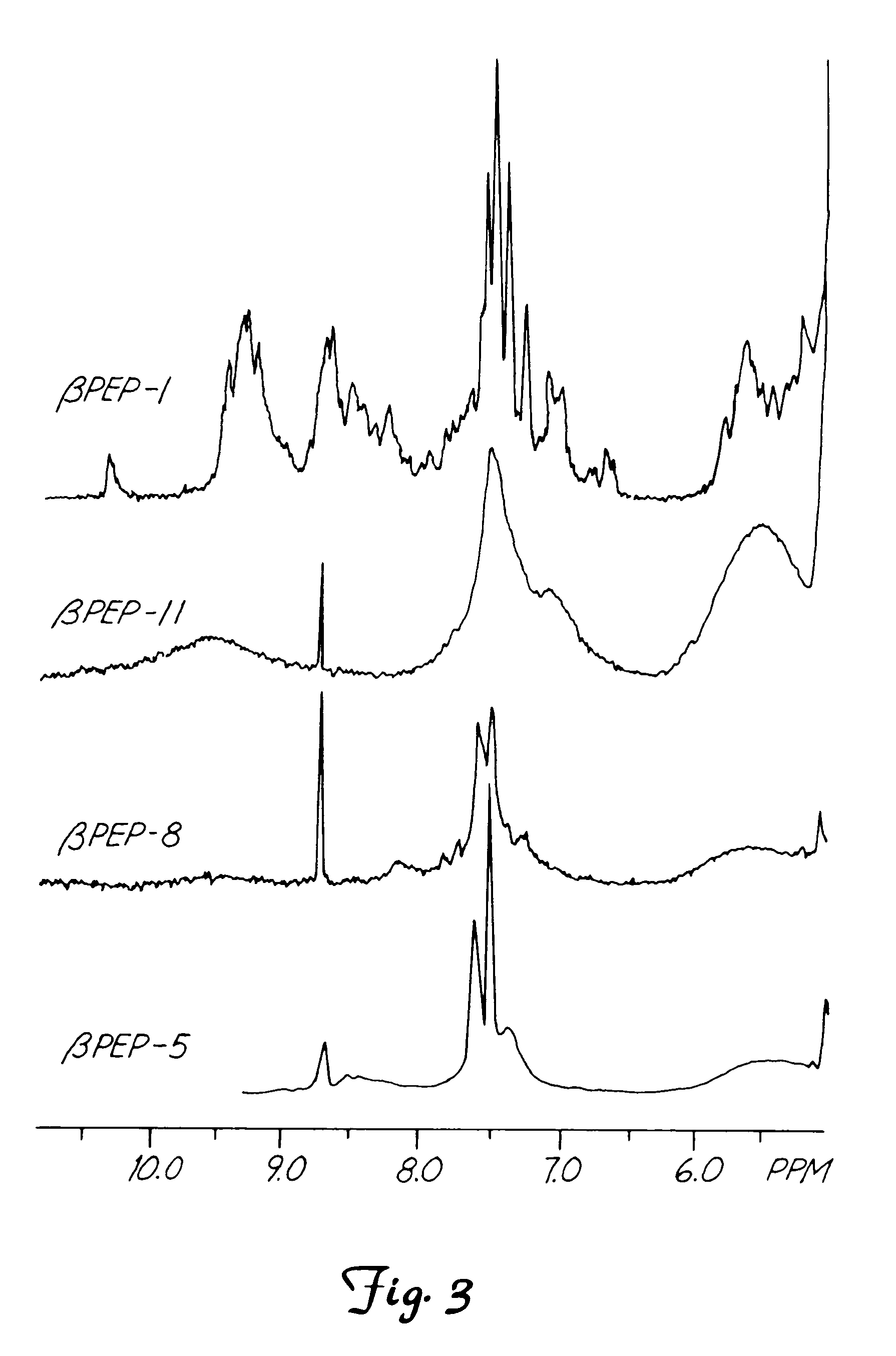
This invention relates to the chemical design and production of peptides, peptide structure and three dimensional conformation was assessed using NMR, circular dichroisin and pulsed field gradient NMR. In addition, this invention relates to peptides produced by these methods and to methods for using the peptides.
Owner:RGT UNIV OF MINNESOTA
Purification of a Drug Substance of a Factor VII Polypeptide by Removal of DesGla-Factor VII Polypeptide Structures
InactiveUS20090042784A1Peptide/protein ingredientsMammal material medical ingredientsIon exchangeElution
The present invention relates to a purification process for drug substances of a Factor VII polypeptide having an impurity in the form of desGla-Factor VII polypeptide structures. The process utilizes an anion-exchange material and includes washing and / or elution with a buffer of a predetermined pH.
Owner:NOVO NORDISK AS
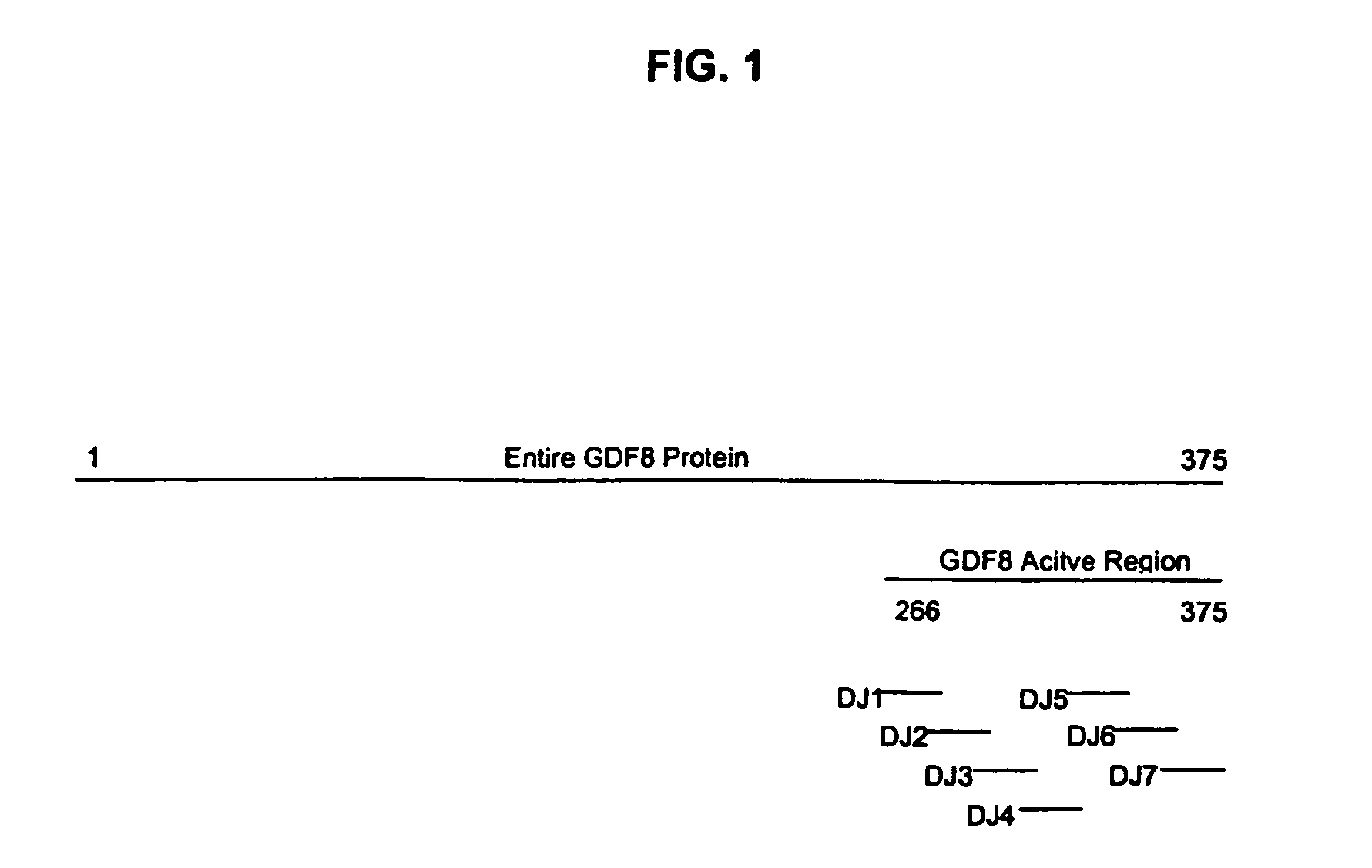
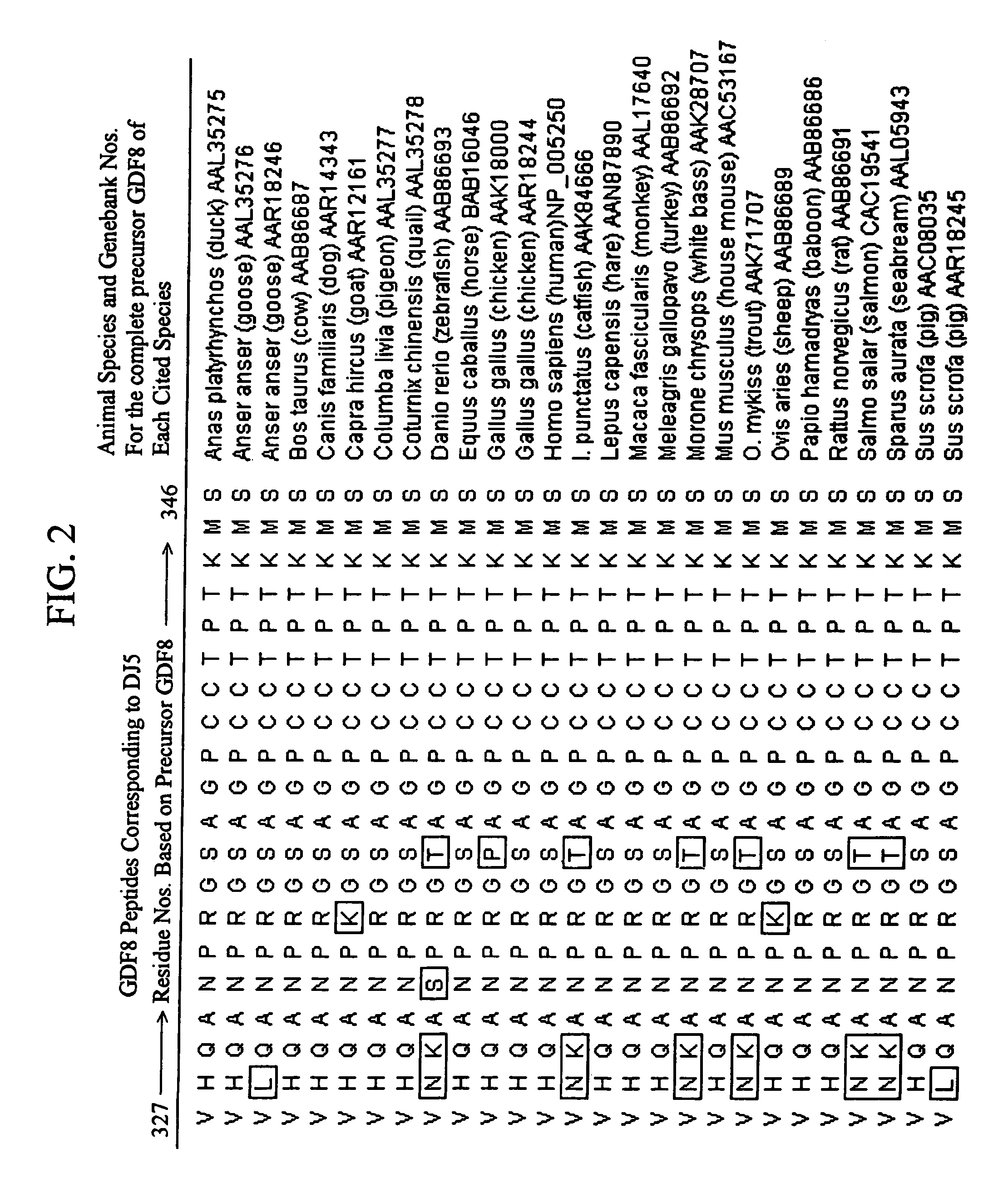
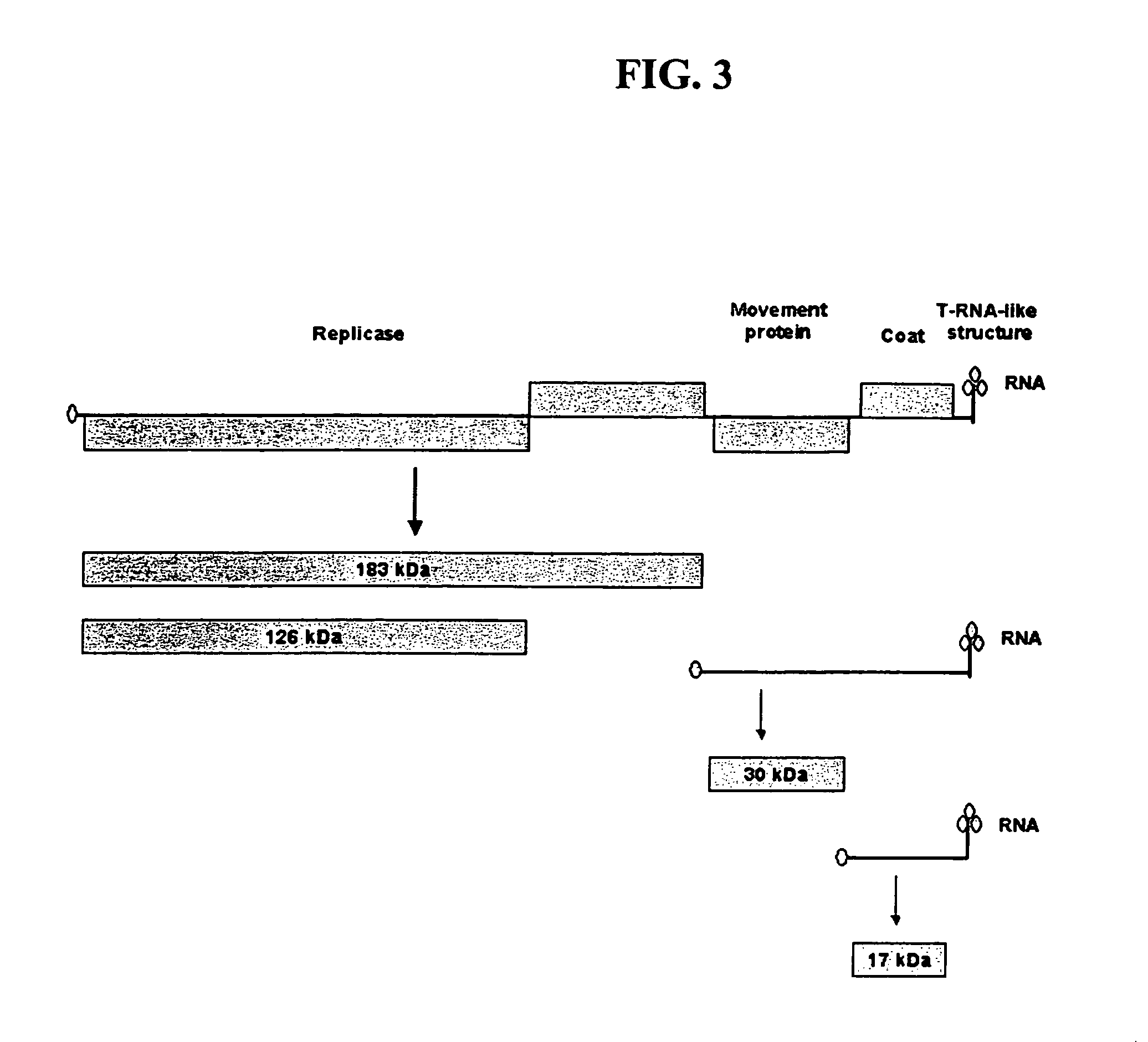
Plant virus coat fusion proteins with GDF8 epitopes and vaccines thereof
Owner:SCHERING PLOUGH ANIMAL HEALTH +1
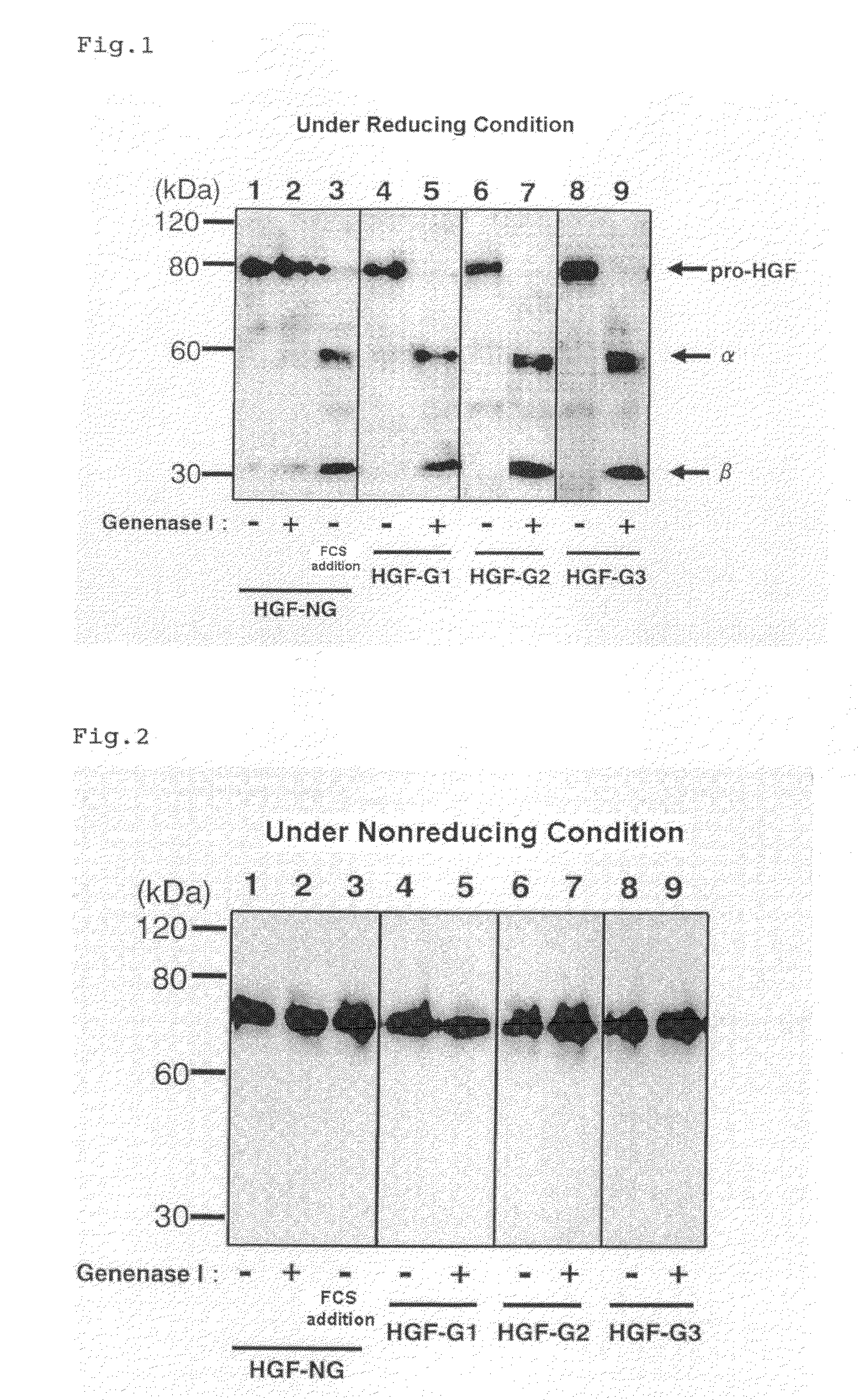
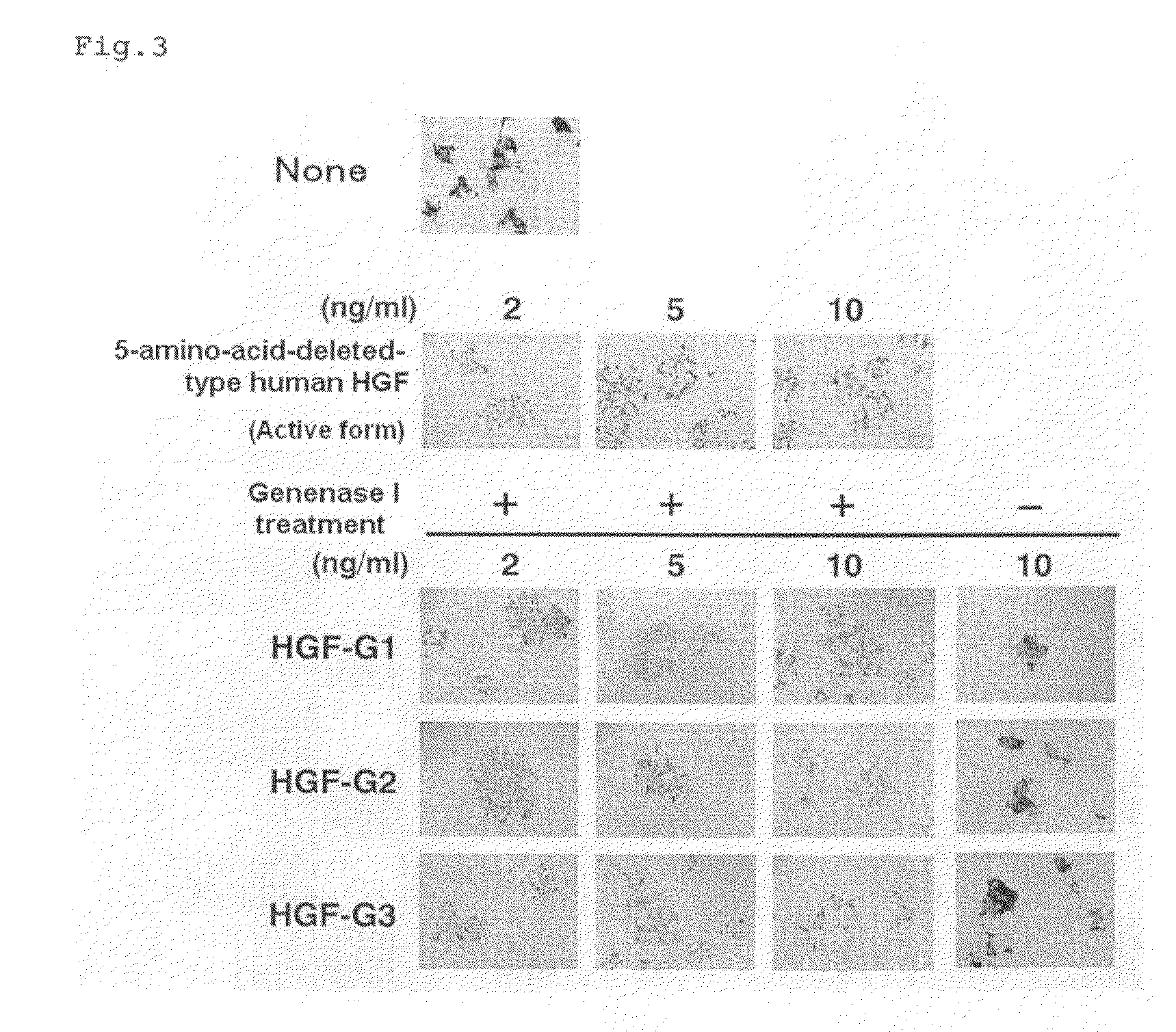
Hgf Precursor Protein Variant and Active Protein Thereof
ActiveUS20090209463A1Low cost productionAvoid contamination riskAntibacterial agentsSenses disorderCrystallographyChemical reaction
An HGF precursor protein variant, in which a peptide structure comprises a sequence including a peptide chain X inserted between an α chain of HGF or a polypeptide where 1 to 20 amino-acid residues from the C-terminus of theα chain are deleted, and a β chain of HGF or a polypeptide where 1 to 20 amino-acid residues from the N-terminus of the β chain are deleted; wherein (i) the peptide chain X has an amino-acid sequence of at least two residues, (ii) the peptide chain X can be cleaved by a protease reaction or a chemical reaction, and (iii) a protein obtained by cleaving at least one site of the peptide chain X has HGF action.
Owner:OSAKA UNIV +1
Primer, primer set, and nucleic acid amplification method and mutation detection method using the same
ActiveUS8067162B2Strong fluorescenceWeak fluorescenceMethine/polymethine dyesSugar derivativesFluorescenceAtomic group
The present invention provides a primer that effectively can detect, for example, the double helix structure of a nucleic acid. The primer is a labeled nucleic acid containing at least one structure represented by the following formula (16),where B is an atomic group having a nucleobase skeleton, E is an atomic group having a deoxyribose skeleton, a ribose skeleton, or a structure derived from either one of them, or an atomic group having a peptide structure or a peptoid structure, and Z11 and Z12 are each an atomic group that exhibits fluorescence and are identical to or different from each other.
Owner:DNAFORM
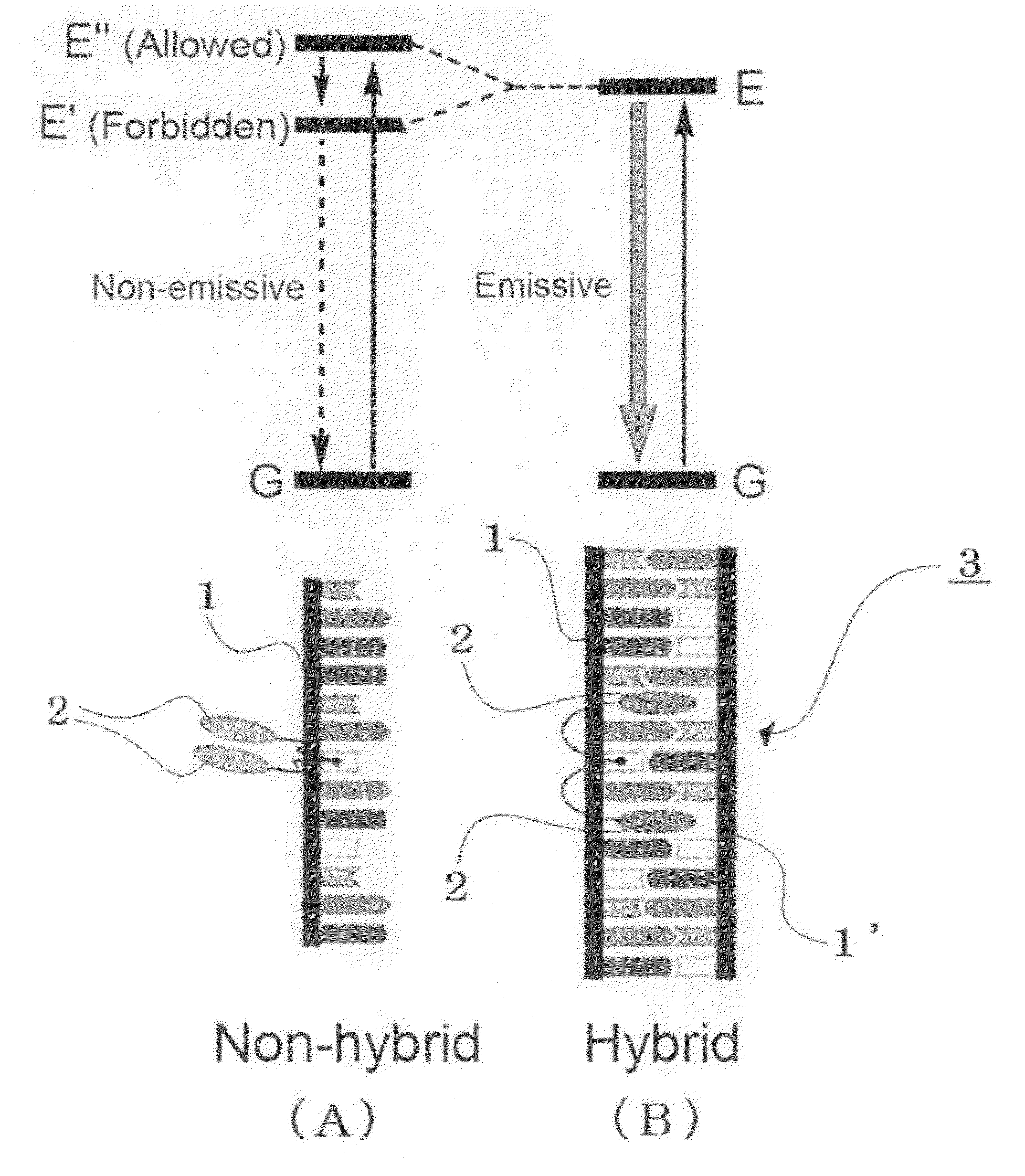
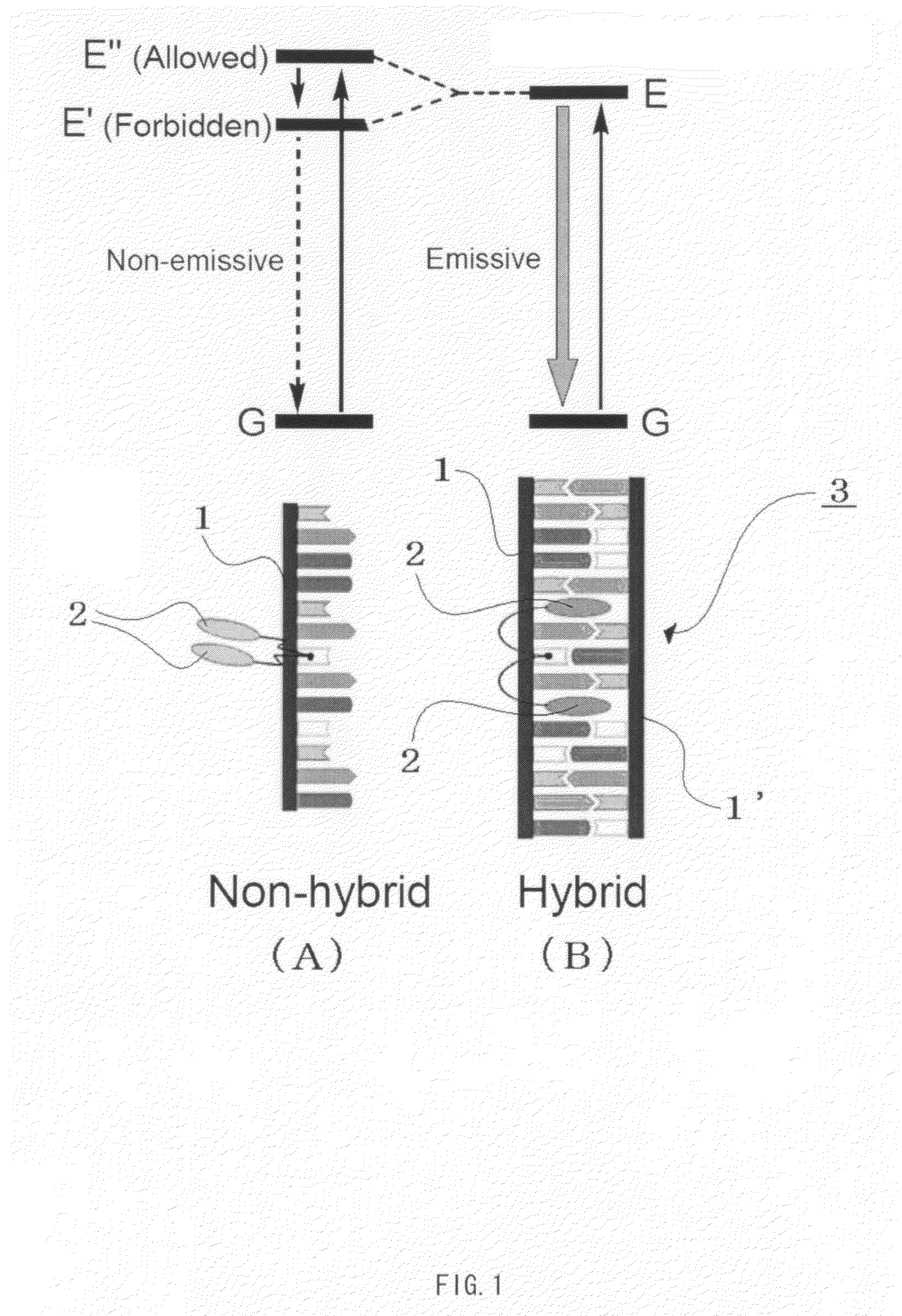
Cyclodextrin dimers with spacers having peptide structures for encapsulation of pharmaceutically active substances with potential high side-effects
InactiveUS20020094950A1Safely and efficiently achieveHighly effectiveOrganic active ingredientsHydrolasesHuman useSide effect
A rigidly spaced, cyclodextrin dimers having a preselected breaking point within the spacer sequence so as to controllably release the active pharmaceutically active substance only after it reaches the desired treatment site is described. These preselected breaking points are stable in blood but are cleavable within cells. In preferred embodiments, the cyclodextrin-pharmaceutically active substance complex is targeted to specific sites via incorporation of specific antibodies for the targeted sites, typically by complexing a biotin-avidin system to specific antibodies which thereby targets the complex to a specific site. Once at the site as the complex is taken up into the cell the preselected break point is cleaved and the encapsulated pharmaceutically active substance becomes available for action within the targeted cell. This approach permits the use of highly effective and efficient pharmaceutically active substances, whose safety restricts use to last chance efforts or which are unable to qualify for human use due to their potential side effects. In a preferred embodiment peptide structures are used as part of the spacers between bridged cyclodextrins The cyclodextrin oligomers are complexed with pharmaceuticals with potential high side effects to safely, efficiently achieve the therapeutic action dsired.
Owner:BIOLITEC PHARMA MARKETING
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979AMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticL929 cellEscherichia coli
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Transglutaminase conjugation method with a glycine based linker
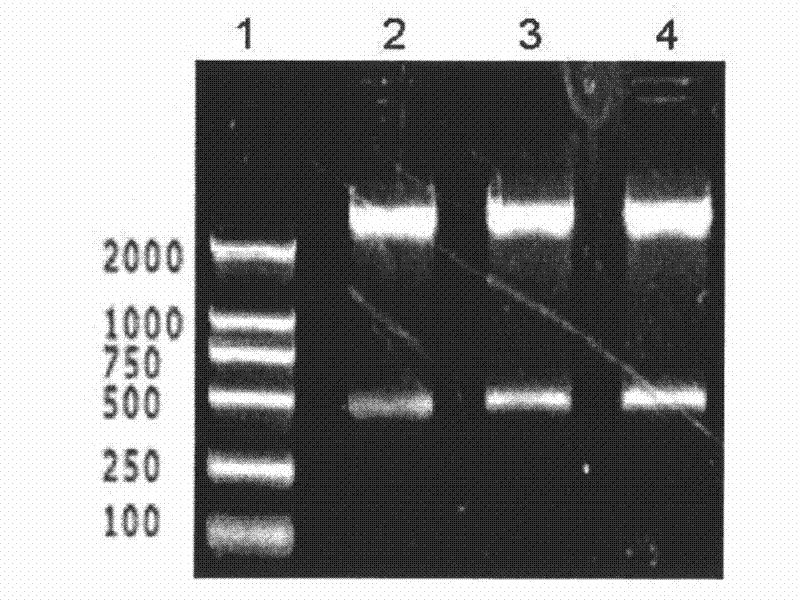
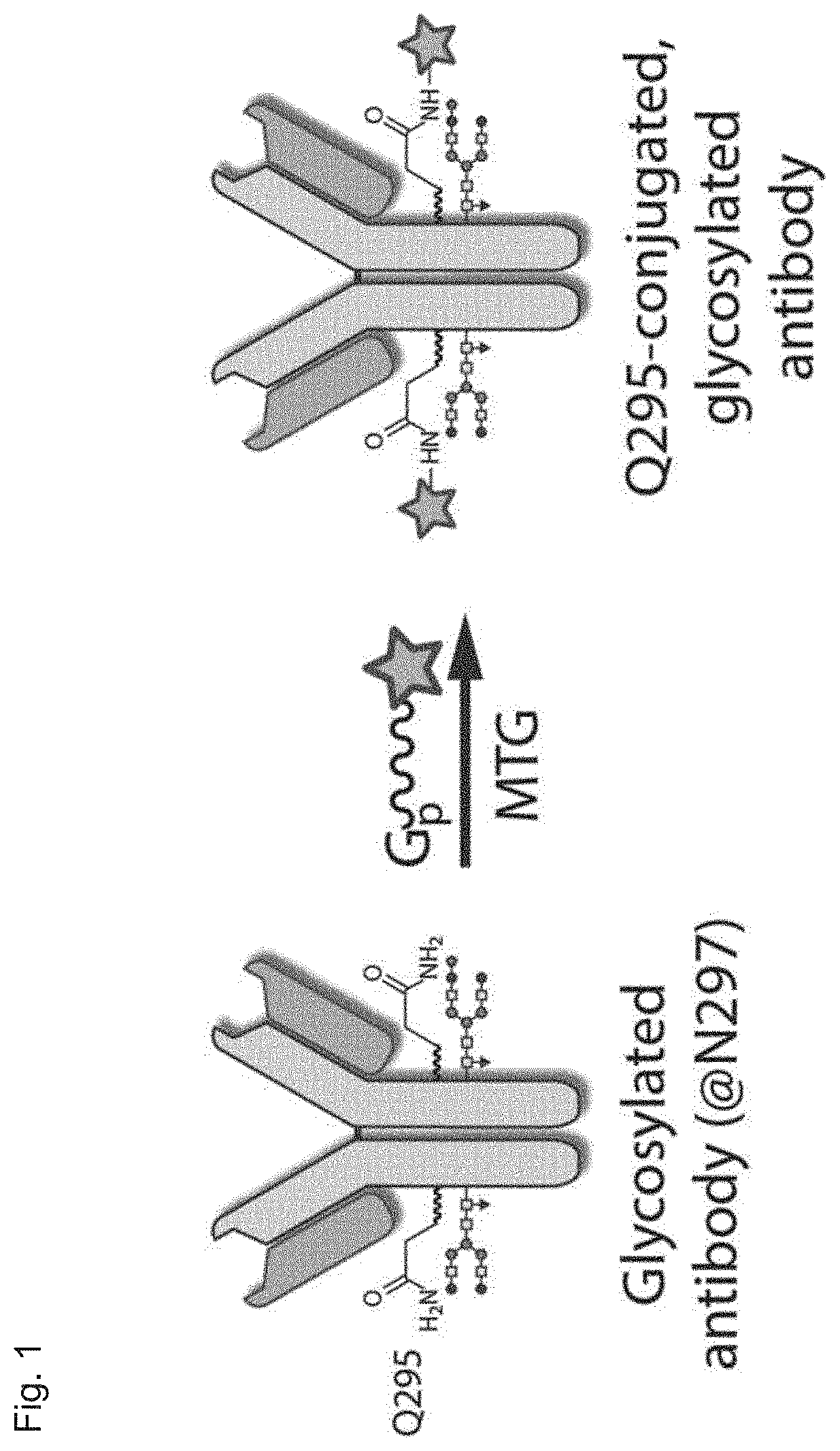
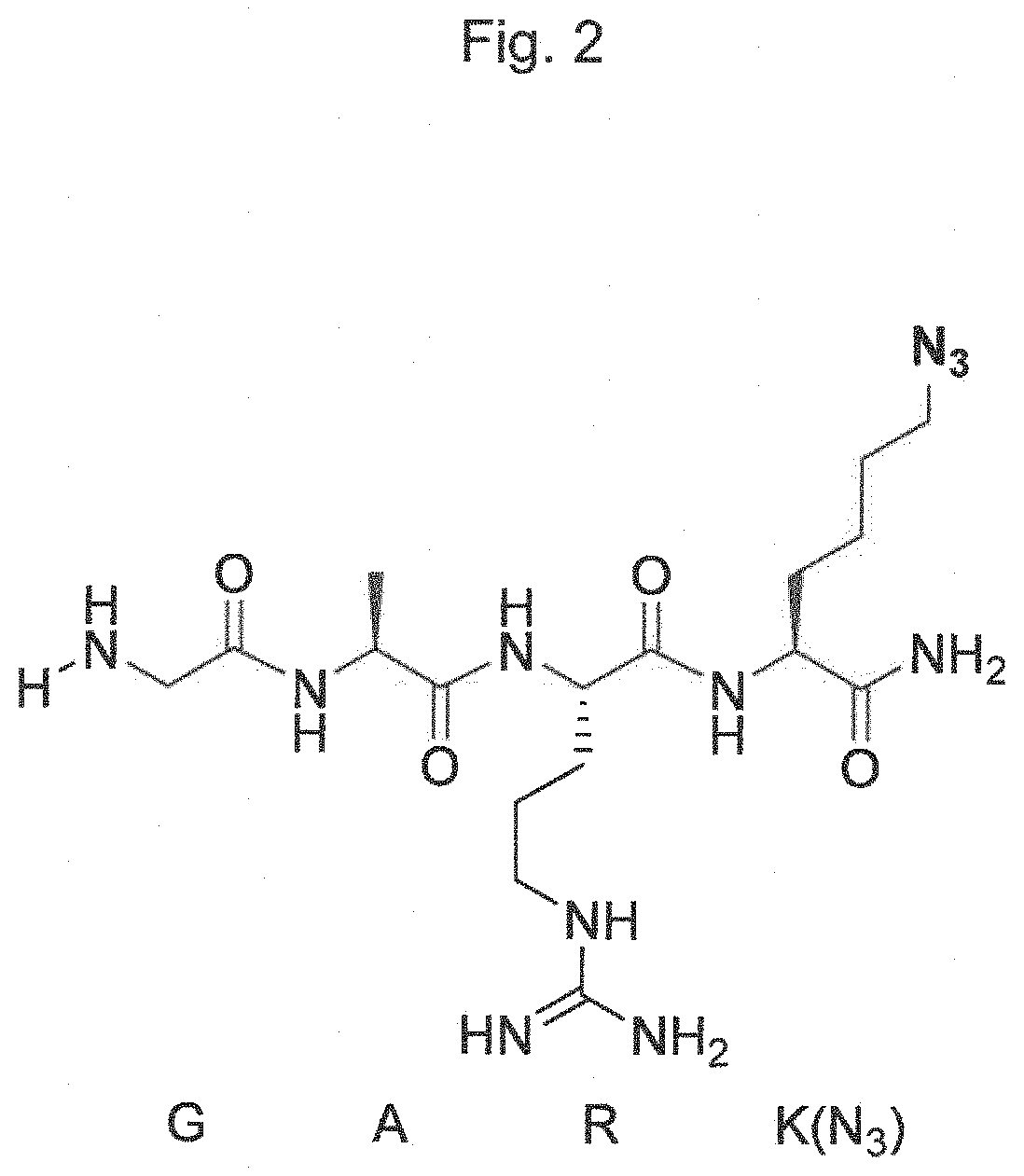
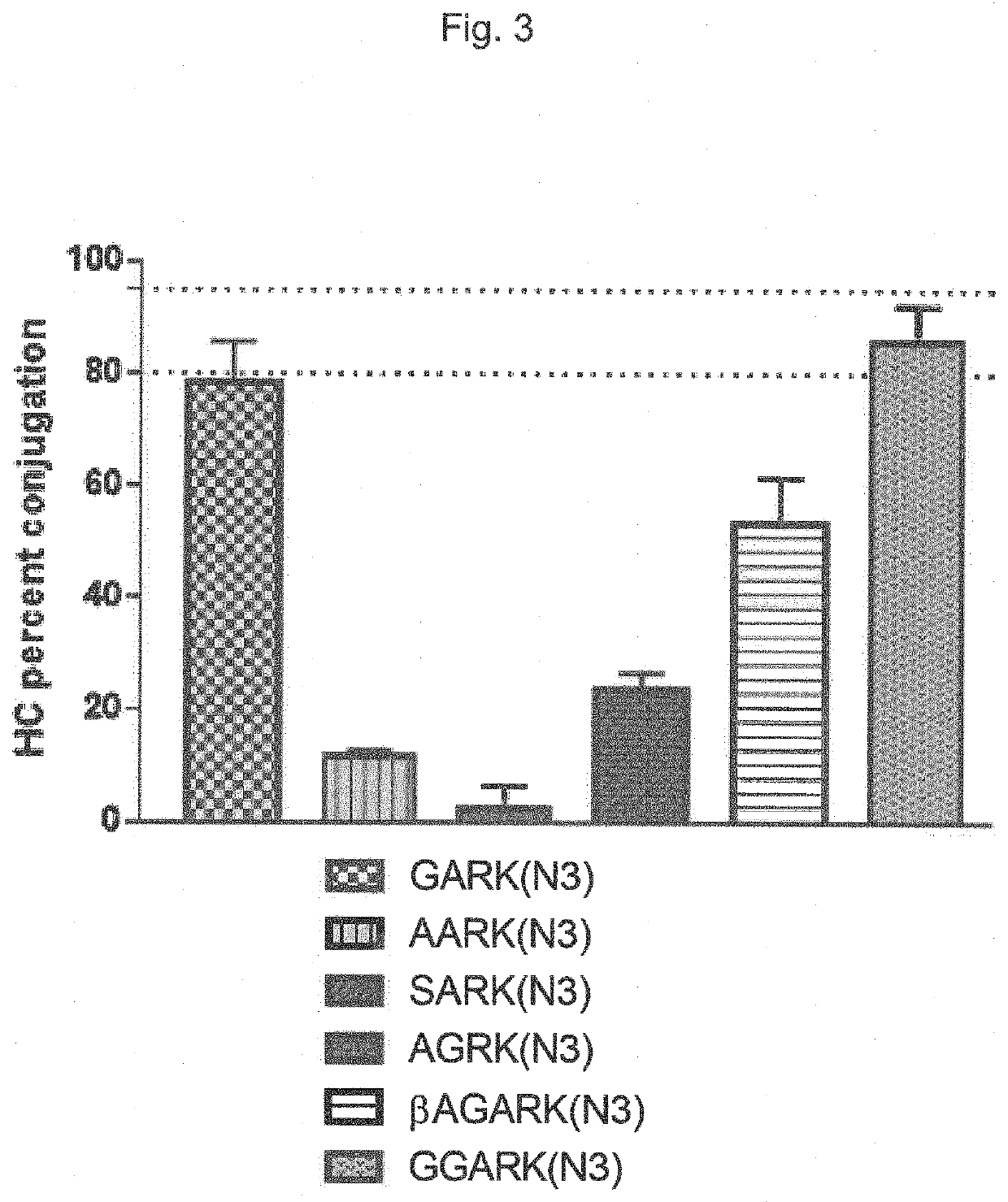
The present invention relates to a method for generating an antibody-payload conjugate by means of a microbial transglutaminase (MTG). The method comprises a step of conjugating a linker comprising or having the peptide structure (shown in N->C direction) Gly-(Aax)m-B-(Aax)n via the N-terminal primary amine of the N-terminal glycine (Gly) residue to a glutamine (Gln) residue comprised in the heavy or light chain of an antibody.
Owner:PAUL SCHERRER INSTITUT
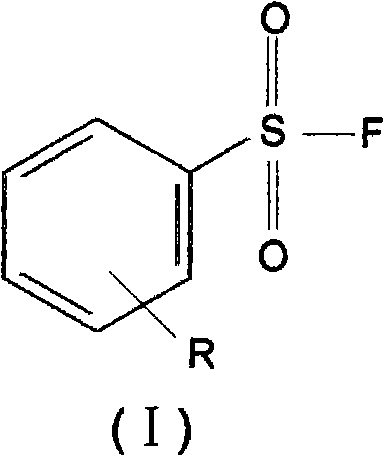
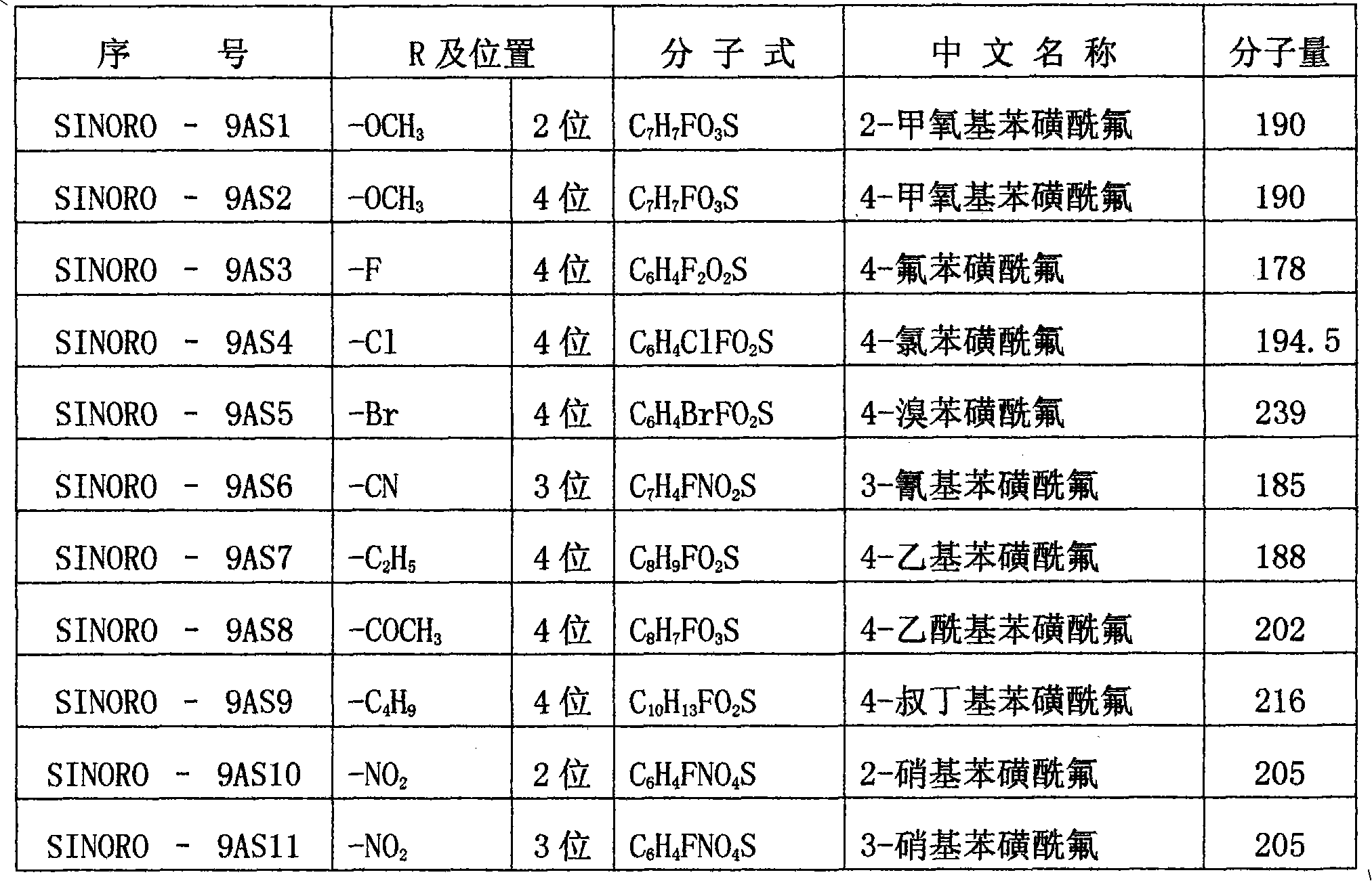
Benzenesulphonyl fluoride, preparing method and application thereof
InactiveCN101585787ASmall molecular weightImprove permeabilityPeptide preparation methodsBiological testingDiseaseBenzenesulfonyl chloride
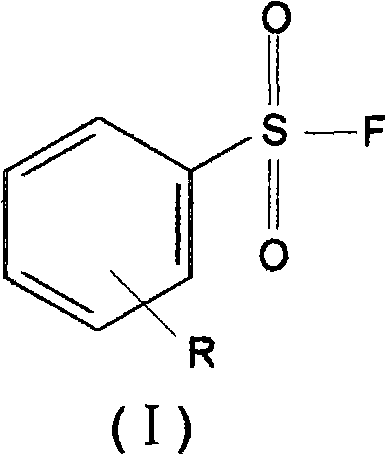
The present invention relates to benzenesulphonyl fluoride, a preparing method and an application thereof. The invention relates to the compound with the general formula of (I), wherein R is alkyl, alkoxyl, acyl, carboxyl, cyano-group, nitryl, halogen or the combination of the groups. The compound is prepared through executing fluorination in the ethane nitrile while the precursor compound of benzene sulfochloride and the metal fluoride are used as raw materials. The new kind of benzenesulphonyl fluoride which is prepared thorugh using the sulfuryl fluoride (-SO2F) group as the reacting group, the parent benzene ring as a chromosphere and the R as the auxiliary substituent group not only can be used for measuring the protein, the peptide structure and the amino acid and researching the protein or the protemics, but also can be used for developing the new medicine or used for diagnosing the difficult and complicated disease in the medical health agency. Furthermore the benzenesulphonyl fluoride of the invention can also be used as a marking agent for quality monitoring by the national medicine checking agency and the quality supervision department.
Owner:李寿椿
Peptide substrates recognisable by a botulinum toxin A, BoNT/A and the use thereof
InactiveCN1969046AQuick checkHigh sensitivityMicrobiological testing/measurementDepsipeptidesCrystallographyPeptide substrate
The invention relates to a peptide substrate selectively recognisable by a botulinum toxin A, BoNT / A comprising a Nop-(Z)-Pya fragment in the peptide structure thereof, wherein Z is an aminoacid chain, preferably RA and said fragment is cleaved by the toxin. Said invention also relates to the substrate use, in particular for carrying out methods for detecting, identifying and / or dosing the botulinum toxin A at very low concentrations (of order of 20 pg) or the inhibitors and / or activators of the toxin metallopeptidase activity, thereby making it possible to quantify the power thereof.
Owner:PHARMALEADS SA
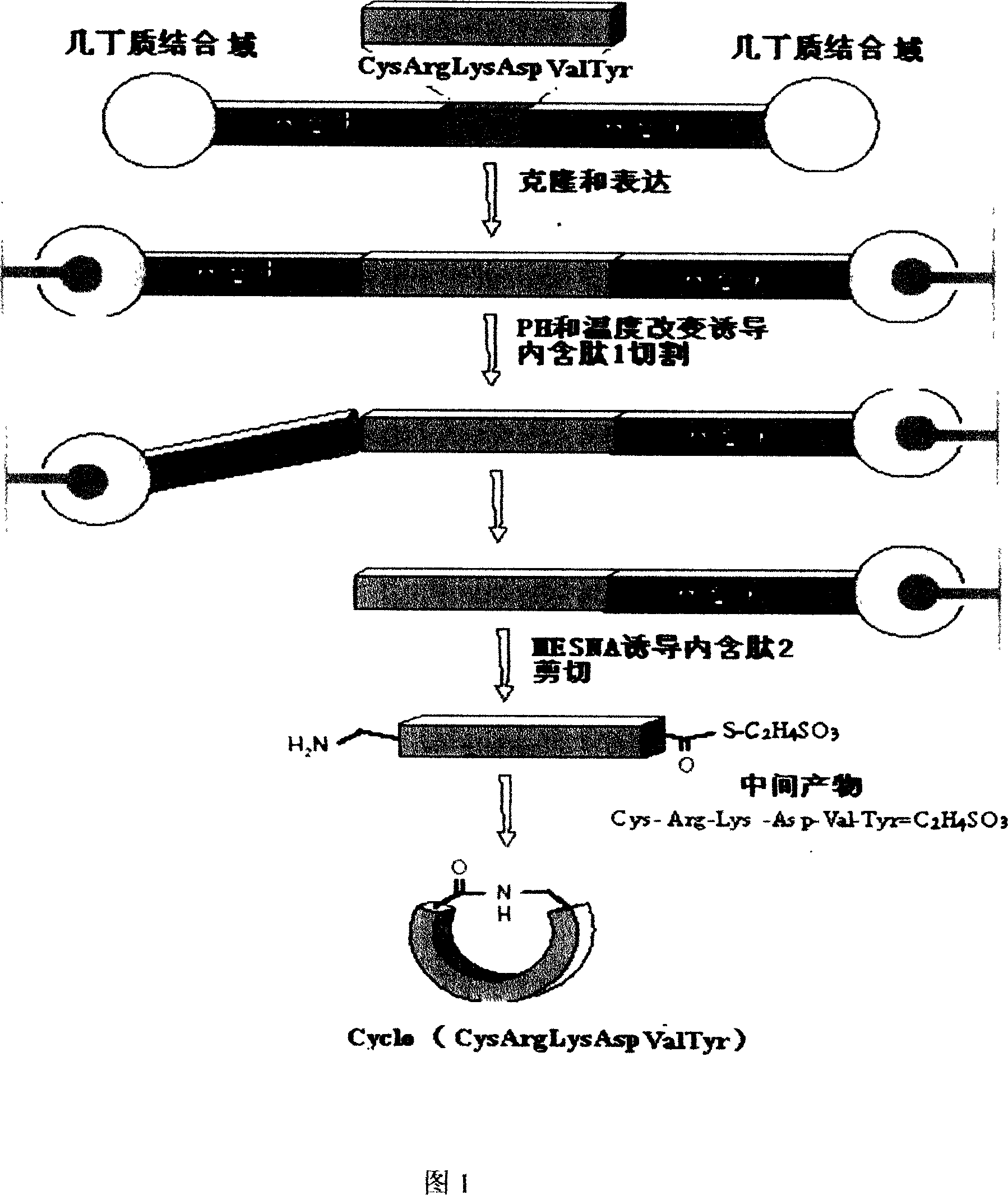
Recombinant thymus pentapeptide structural analogs, its production and use
Recombinant thymus pentapeptide structural analogs, its production, expression and use for preparing related immune diseases medicines are disclosed. The structural formula is cyclo-(Cys-Arg-Lys-Asp-Val-Tyr-), and it consists of Cys-guided circular thymic peptide and 6 amino acids. It has better biological activity and stability.
Owner:广州达信生物技术有限公司
RAGE fusion proteins and methods of use
InactiveCN101410411AMetabolic stabilityInhibit bindingAntibacterial agentsNervous disorderHigh affinity bindingChemistry
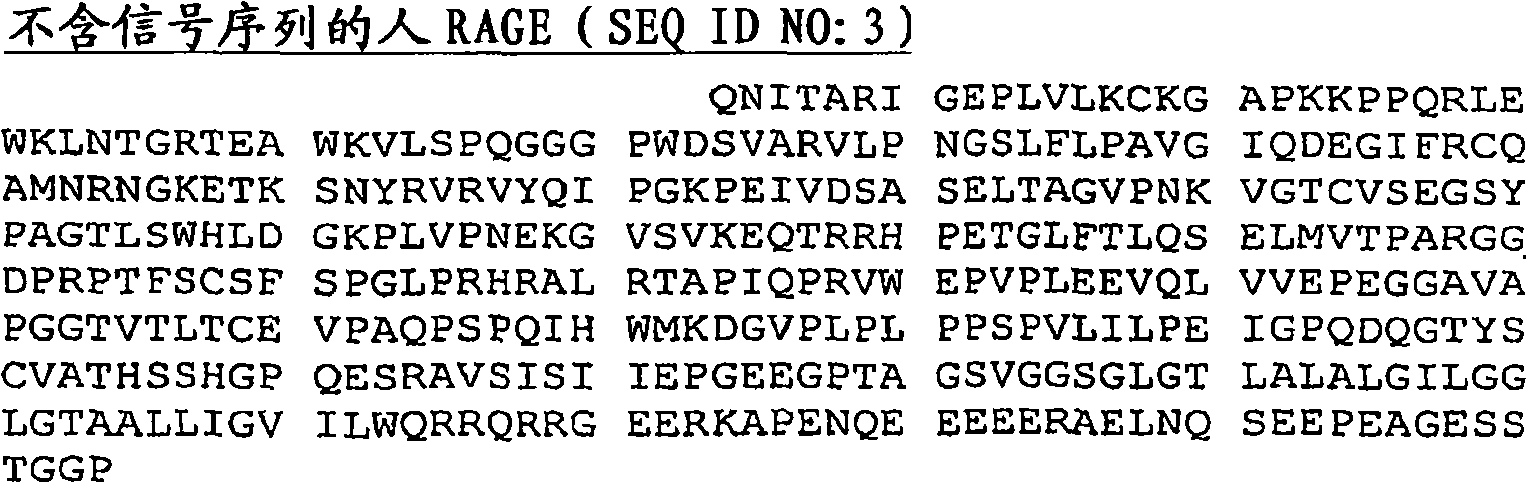
Disclosed are RAGE fusion proteins comprising RAGE polypeptide sequences linked to a second, non-RAGE polypeptide. The RAGE fusion protein may utilize a RAGE polypeptide domain comprising a RAGE ligand binding site and an interdomain linker directly linked to an immunoglobulin CH2 domain. Such fusion proteins may provide specific, high affinity binding to RAGE ligands. Also disclosed is the use of the RAGE fusion proteins as therapeutics for RAGE-mediated pathologies.
Owner:TRANSTECH PHARMA
Synthetic library of specific binding molecules
ActiveCN105492610AGeneral/multifunctional contrast agentsBiological material analysisA-DNAProstate-specific antigen
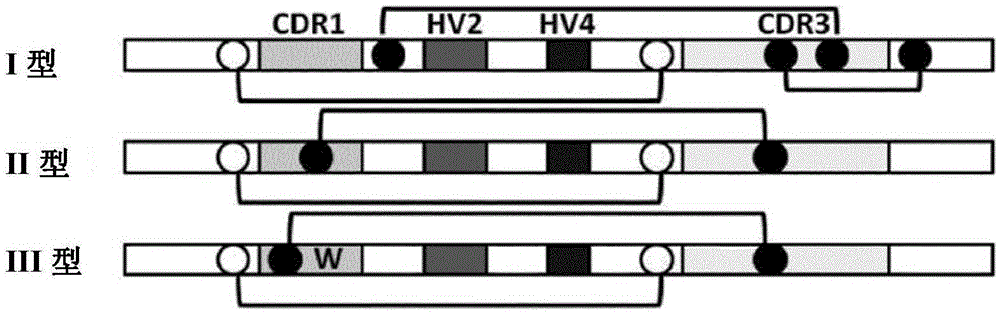
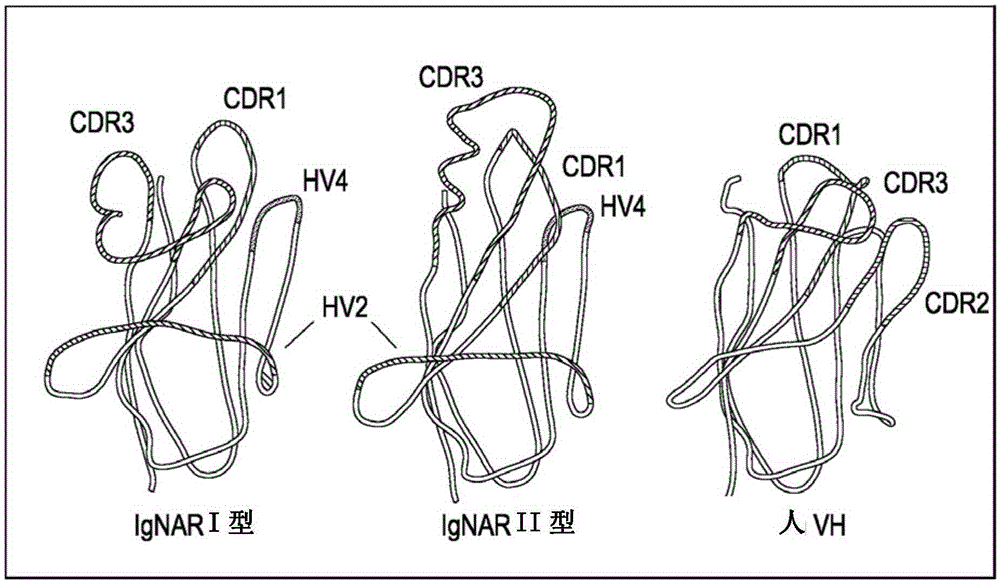
The present invention provides methods for the production of a library of antigen specific antigen binding molecules having a peptide domain structure represented by the following formula (I): FW1-CDR1-FW2-HV2-FW3a-HV4-FW3b-CDR3-FW4 comprising (1) isolating RNA from a member of a species in the Elasmobranchii subclass; (2) amplifying DNA sequences from RNA obtained; (3) selecting a DNA sequence from the database prepared; (4) amplifying DNA sequences encoding two or more contiguous peptide domains of FW1-CDR1-FW2-HV2-FW3a-HV4-FW3b-CDR3-FW4; (5) ligating together said amplified DNA sequences to form DNA sequences encoding an antigen specific binding molecule; (6) cloning the amplified DNA obtained into a display vector; and (7) transforming a host with said display vector to produce a library of said antigen specific antigen binding molecules. The invention also provides methods for the production of an antigen specific antigen binding molecule as defined, pharmaceutical compositions comprising such molecules and uses thereof in medicine.
Owner:艾莱斯摩根公司
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979BMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticEscherichia coliL929 cell
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for preparing high-efficiency antioxidant potato protein antioxidant peptide
InactiveCN101824458AReduce oxidationIncrease industrial added valuePeptide preparation methodsFermentationSephadexEmulsion
The invention relates to a method for preparing a high-efficiency antioxidant potato protein antioxidant peptide, and belongs to the technical field of fine and deep processing of agricultural products. A novel and high-efficiency potato protein antioxidant peptide product is prepared from industrial potato proteins serving as raw materials by the steps of Alcalase hydrolysis, enzymolysis liquid microfiltration concentration, Sephadex G15 gel column chromatography, freeze drying and the like, wherein peptide structures are YEF (Tyr-Phe-Glu), NYKQM (Asn-Tyr-Lys-Gln-Me) and TY (Thr-Tyr) respectively; and the range of relative molecular weight of the antioxidant peptide is between 100 and 1043Da. The product prepared by the method has relatively strong free radical scavenging capacity and high safety, and can effectively improve the oxidation stability of fat in an emulsion; and the preparation method is simple, can effectively improve the functional properties of the potato proteins, and has important significance for the deep processing and the added value increase of the potato proteins and the comprehensive utilization and the extension of an industrial chain of potatoes.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com