RAGE fusion proteins and methods of use
A technology of fusion protein and protein, which is applied in the direction of fusion polypeptide, chemical instruments and methods, and diagnosis by using light, which can solve the problem of sRAGE short half-life and achieve the effect of improving diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0262] Example 1A: Production of RAGE fusion proteins
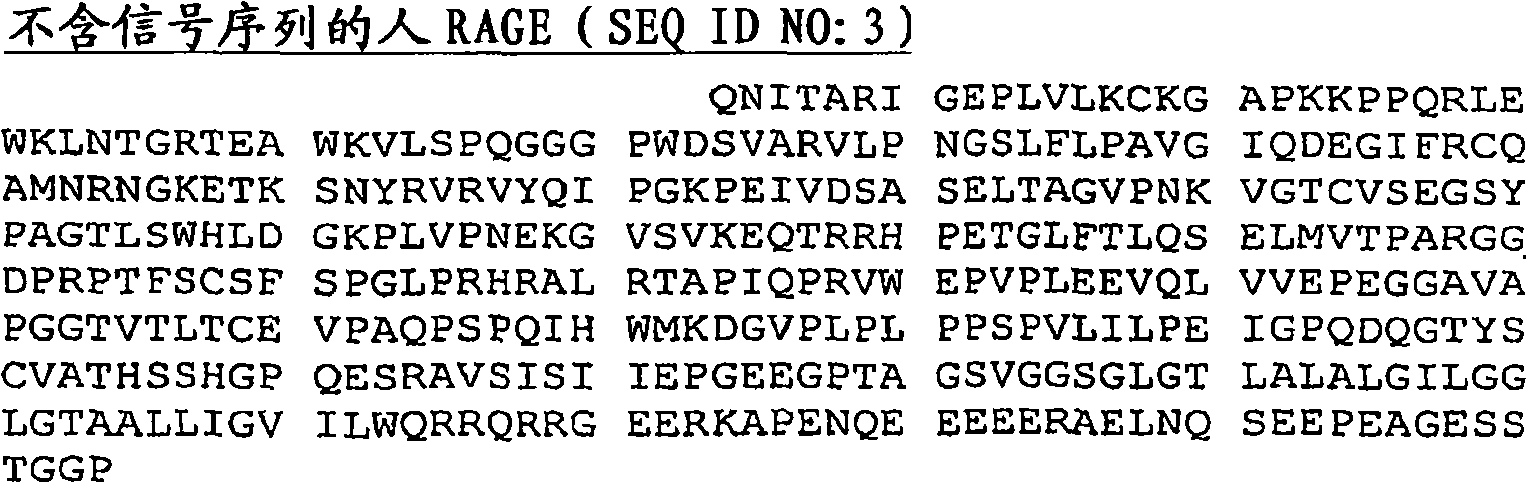
[0263] Two plasmids were constructed for expressing RAGE-IgG fusion protein. Both plasmids were constructed by ligating different lengths of 5' cDNA sequences from human RAGE with the same 3' cDNA sequences from human IgG (γ1). These expression sequences (ie ligation products) were then inserted into the pcDNA3.1 expression vector (Invitrogen, CA). figure 2 and 3 The nucleic acid sequence encoding the RAGE fusion protein coding region is shown in . For the TTP-4000RAGE fusion protein, nucleotide sequences 1-753 (highlighted in bold) encode the RAGE N-terminal protein sequence, while nucleotide sequences 754-1386 encode the IgG protein sequence ( figure 2 ). For TTP-3000, nucleotide sequences 1-408 (highlighted in bold) encode the RAGE N-terminal protein sequence, while nucleotide sequences 409-1041 encode the IgG protein sequence ( image 3 ).
[0264] To produce the RAGE fusion protein, an expression vector com...
Embodiment 1B
[0265] Example 1B: Production of RAGE fusion proteins
[0266] A plasmid was constructed for expressing RAGE-IgG fusion protein. This plasmid was constructed by ligating the 5' cDNA sequence from human RAGE with the 3' cDNA sequence from human IgG (γ1). PCR was used to amplify cDNA. In addition, on the 5' end, the PCR primers added an Eco RI restriction enzyme site and a Kozak consensus translation initiation sequence from the cloning process. On the 3' end, the PCR primers added an Xho I restriction site immediately after the end codon. On the 3' end, the PCR primers also included 2 silent base changes that removed a cryptic RNA splice site in the immunoglobulin portion near the end codon. The codon encoding proline (based on residue 409 numbered in the protein sequence in SEQ ID NO: 32) was changed from CCG to CCC, and the codon encoding glycine (based on residue 409 in the protein sequence in SEQ ID NO: 32) Numbered residue 410) was changed from GGT to GGG. The PCR f...
Embodiment 2
[0269] Embodiment 2: the method for testing the activity of RAGE-IgG1 fusion protein
[0270] a. In vitro ligand binding:
[0271] Known RAGE ligands were coated on the surface of Maxisorb plates at a concentration of 5 μg / well. Plates were incubated overnight at 4°C. After ligand incubation, the liquid in the plate was aspirated, and a blocking buffer of 50 mM imidazole buffer (pH 7.2) containing 1% BSA was added to the plate for 1 hour at room temperature. Then aspirate the liquid in the plate and / or rinse with washing buffer (20mM imidazole, 150mM NaCl, 0.05% Tween-20, 5mM CaCl 2 and 5mM MgCl 2 , pH 7.2) for washing. A TTP-3000 (TT 3) solution with an initial concentration of 1.082 mg / mL and a TTP-4000 (TT4) solution with an initial concentration of 370 μg / mL were prepared. RAGE fusion protein was added at increasing dilutions of the starting sample. The RAGE fusion protein was allowed to incubate with the immobilized ligand for 1 hour at 37°C, after which the pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com