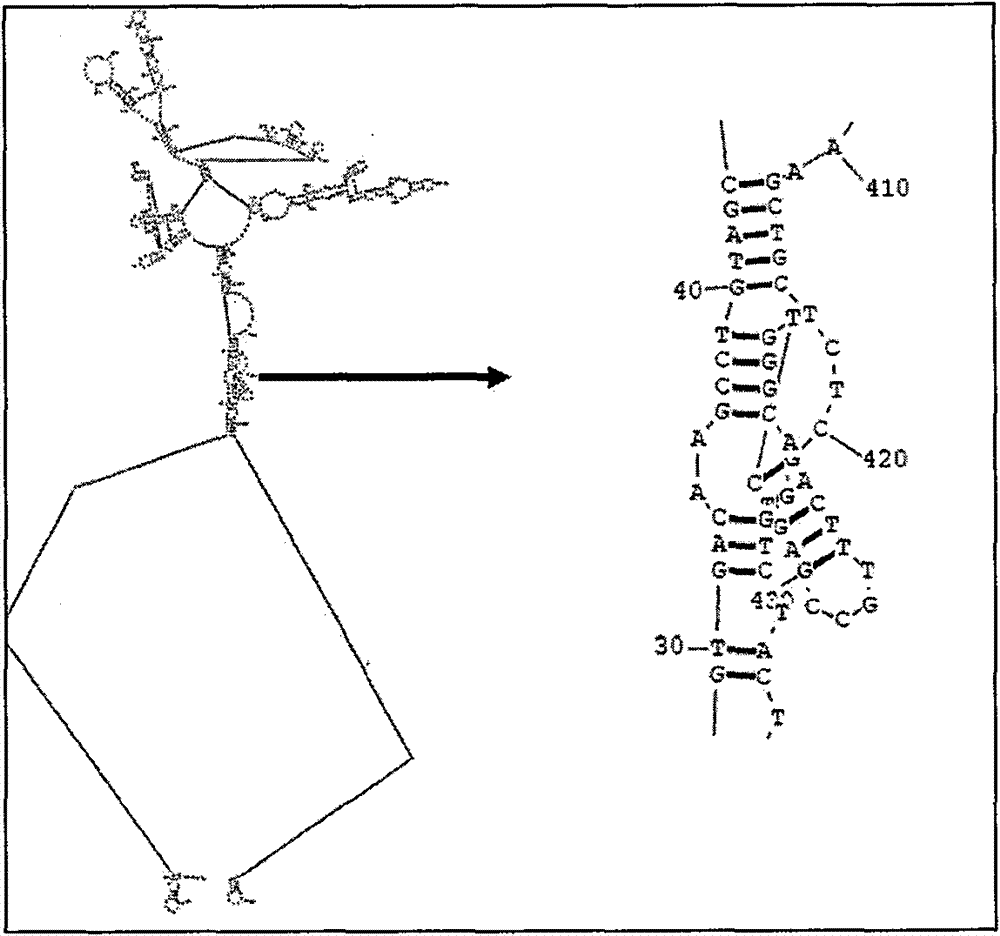
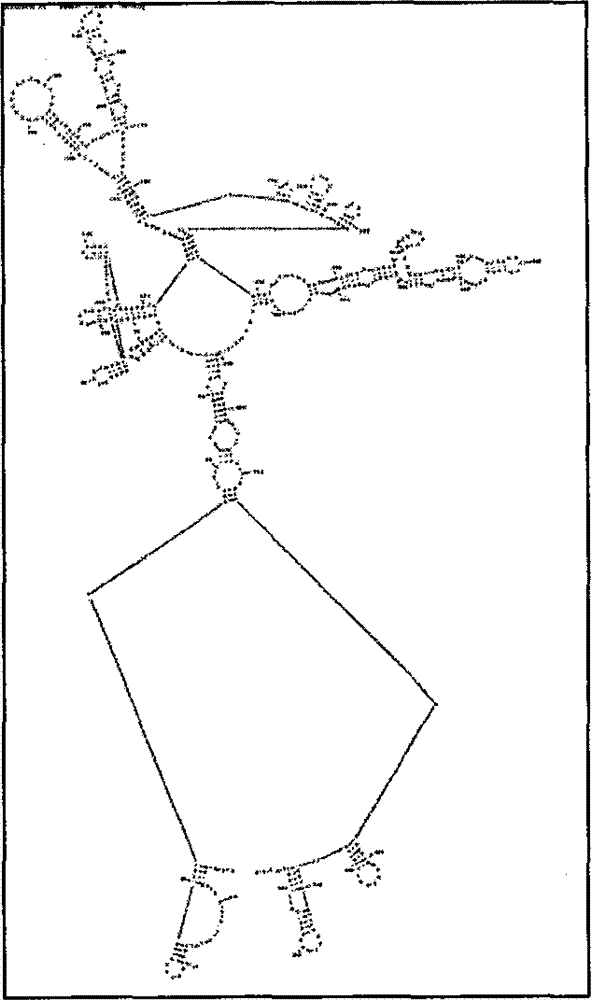
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
A construction method and molecular technology, applied in the field of medicine and biology, can solve problems such as non-neutralization and denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0141] The human TNF therapeutic autovaccine prepared according to the technical scheme of the present invention is encoded with TT 830-844 (QYIKANSKFIGITEL), HEL 46-61 (NTDGSTDYGILQINSR) and PADRE (AKFVAAWT LKA) amino acid nucleic acid sequence to replace human TNF-α129 to 141 amino acid nucleic acid sequence, thereby obtaining hTNF-TT, hTNF-HEL, hTNF-PADRE fusion protein, immunized mice after purification, and compared These three molecules induce the titer of antibodies against hTNF-α and the ability to neutralize the activity of hTNF-α, and hTNF-PADRE has the best immune effect after screening. Immunization of mice with hTNF-PADRE recombinant protein can not only reduce the weight loss of mice with cachexia induced by natural TNF-α, but also prolong the survival period of mice with cachexia induced by mTNF-α; prolong the survival of mice with acute lung injury induced by LPS survival; alleviates symptoms of type II collagen-induced rheumatoid arthritis. Secondly, the rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com