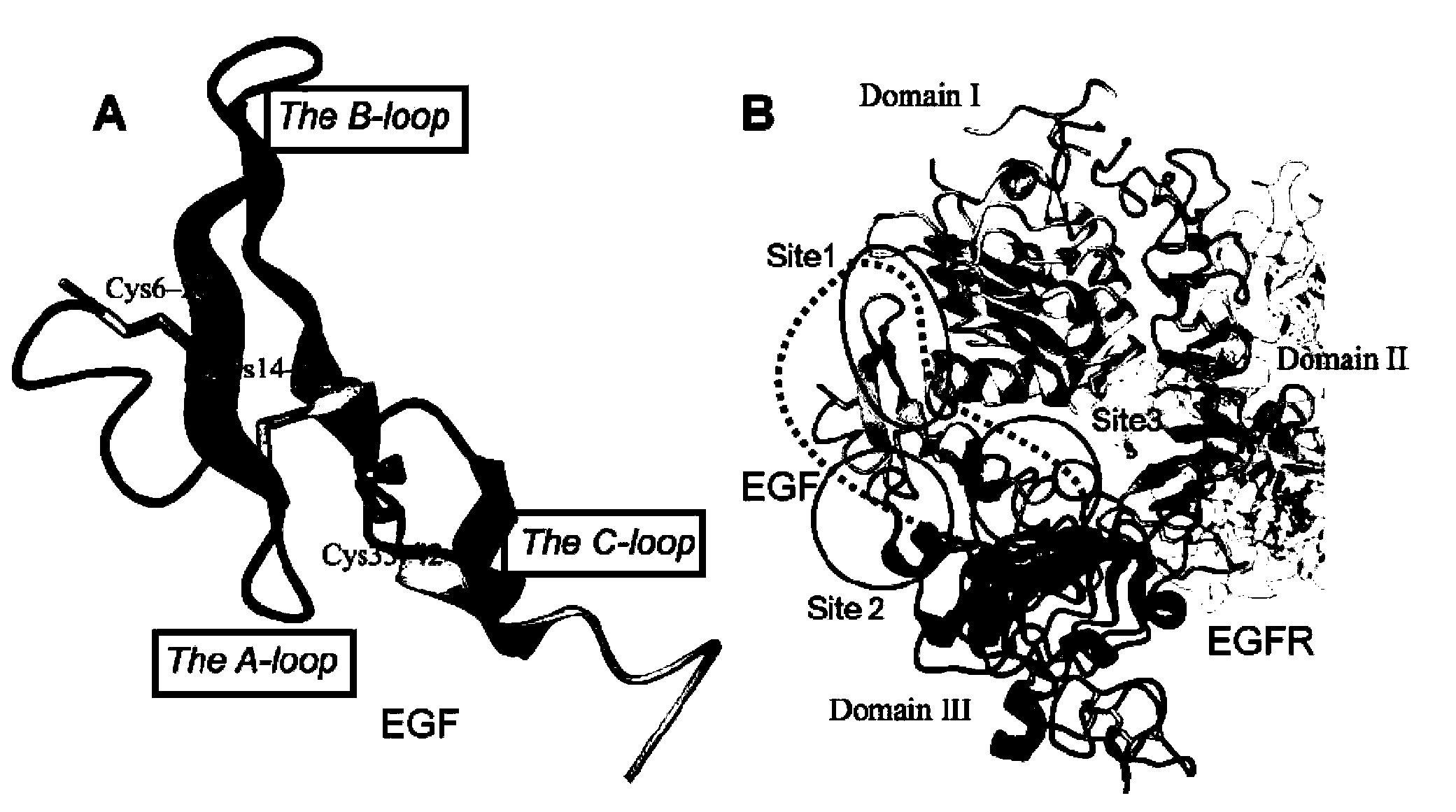
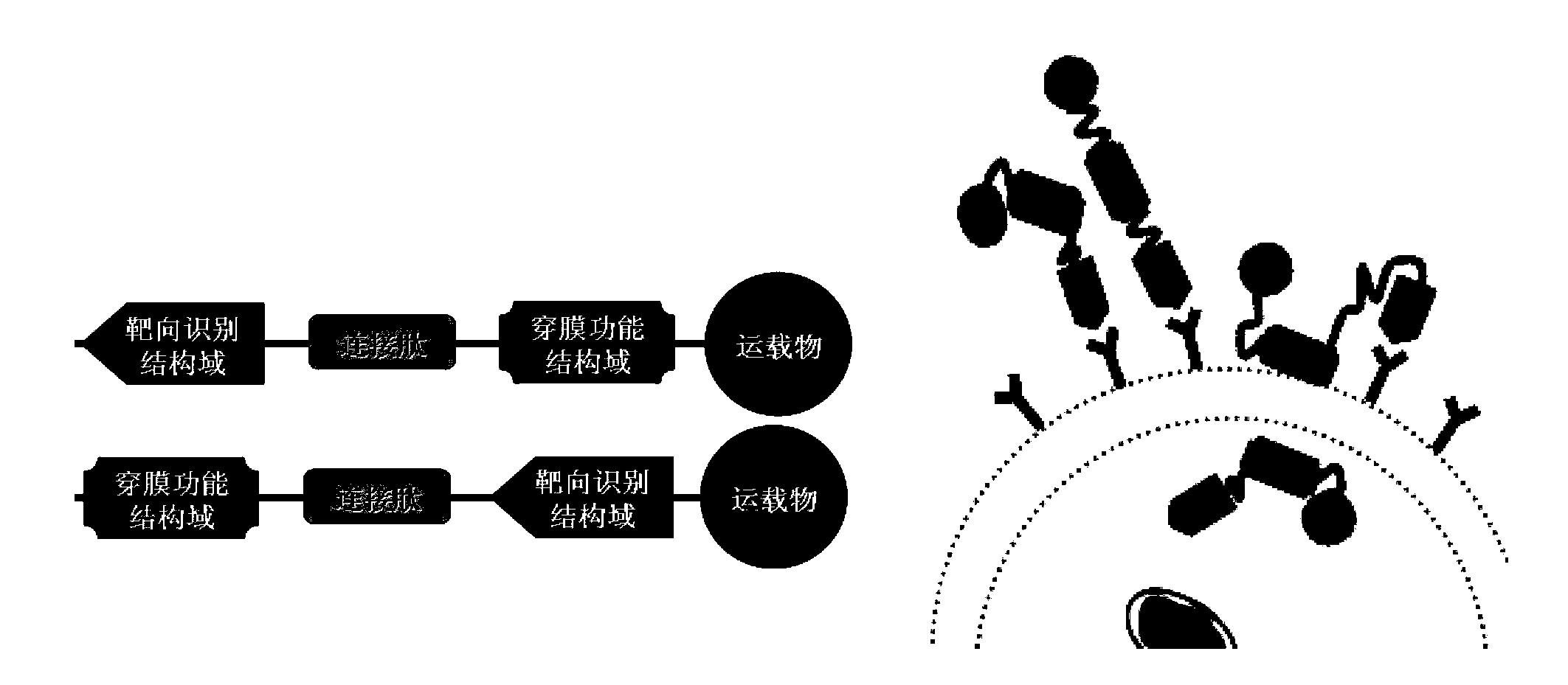
Tumor cell targeting penetrating peptide
A technology of tumor cells and membrane-penetrating peptides, which is applied in the direction of anti-tumor drugs, peptides, and decapeptides, and can solve problems such as affecting biological functions, loss, and reduced binding activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Expression plasmid construction
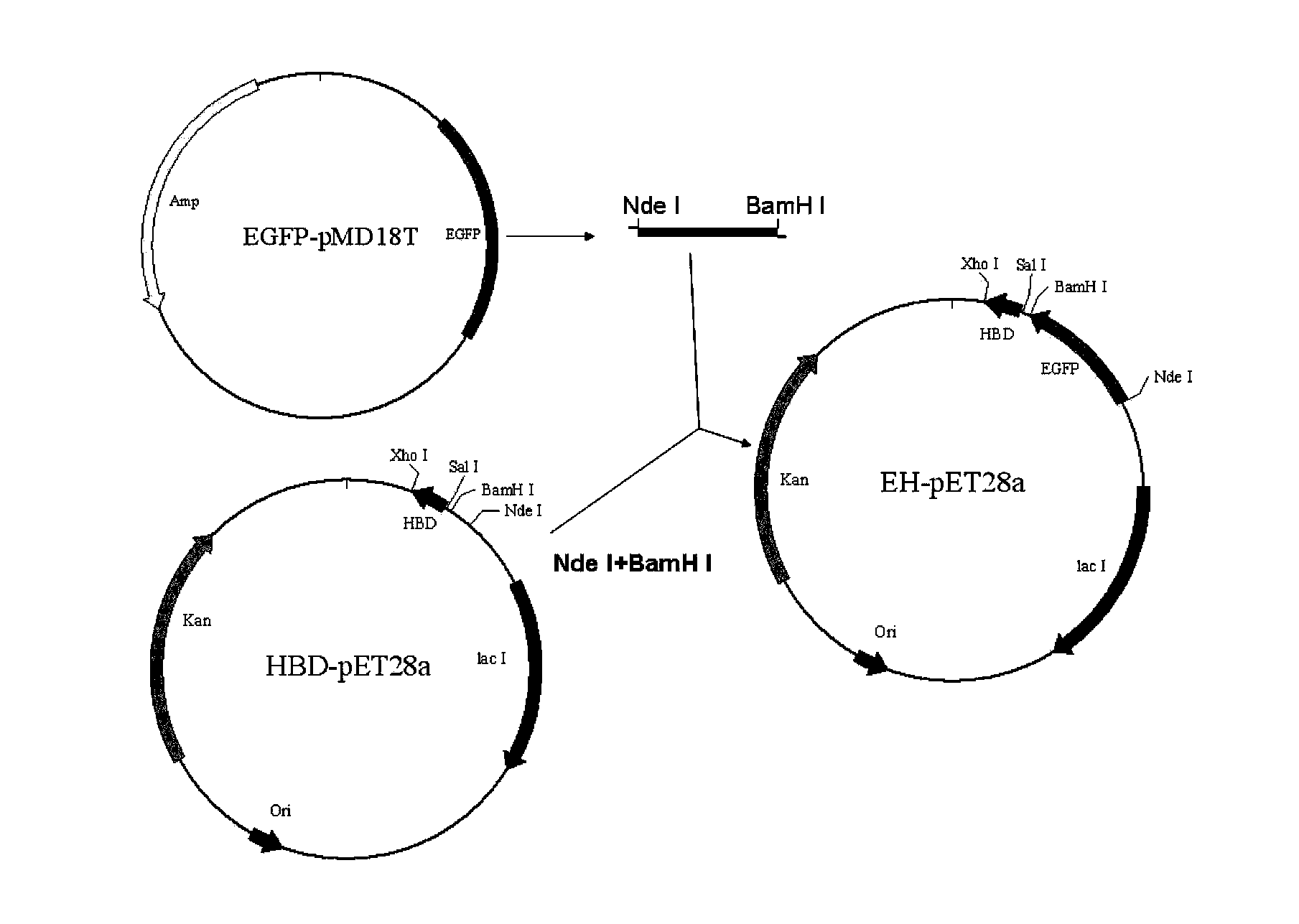
[0101] 1. Construction of EH (recombinant protein with green fluorescent protein-HBD)
[0102] Synthesize primers with Nde I and BamH I restriction sites, carry out PCR amplification of the EGFP gene in the existing EGFP-pMD18T plasmid (Xiao Wei et al., Food and Drugs, 2010, 12(5):158), add Insert restriction sites Nde I and BamH I, insert the amplified fragment into HBD-pET28a, construct EH-pET28a plasmid, referred to as EH, and the expression product is: EGFP-HBD (see image 3 )
[0103] (1) Using EGFP-pMD18T as a template, according to the EGFP gene sequence, design the following primers for PCR amplification. The forward and reverse primers respectively introduce Nde I and BamH I restriction sites:
[0104] Primer 1: 5'-AAA CATATG GTGAGCAAGGGCGAGGAGCTG-3'
[0105] Primer 2: 5'-AAA GGATCC GAGTCCGGACTTGTACAGCTCGTCCATG-3'
[0106] (2) PCR amplification reaction conditions: The reaction conditions of PCR amplification...
Embodiment 2
[0130] Embodiment 2: Recombinant protein expression
[0131] 1. Expression and purification of EH recombinant protein
[0132] (1) Pick a single colony transformed with the EGFP-HBD-pET28a plasmid from the preserved solid LB medium plate and culture it in 3ml LB liquid medium containing kanamycin at 37°C until OD 600 About 0.5.
[0133] (2) Take the bacterial culture solution, insert 1% of the inoculum into a certain volume of medium containing kanamycin and continue to expand the culture until the OD 600 About 0.5-1.0.
[0134] (3) Adjust the culture temperature to 37°C, add an appropriate amount of IPTG to induce the target protein, continue to culture overnight, and collect the bacteria by centrifugation.
[0135] (4) The collected cells were resuspended with 20mM Tris-HCl buffer (pH8.5) and ultrasonically disrupted. Centrifuge to collect the supernatant containing the target protein.
[0136] (5) The collected supernatant was separated by affinity chromatography on a ...
Embodiment 3
[0154] Example 3: Research on the Effect of Penetrating the Membrane
[0155] Incubate ESH recombinant protein in various cells (including tumor cells and normal cells), conduct fluorescence microscope and laser confocal microscope observation and flow cytometry analysis, and use EH recombinant protein and ES recombinant protein as controls to conduct transfusion Membrane activity comparison
[0156] A. Fluorescence microscopy study comparison of transmembrane activity
[0157] (1) HeLa tumor cell line was used to investigate the incubation concentration and incubation time of ESH recombinant protein, and the final concentration of ESH recombinant protein was 40 μg / ml. The experimental results show that the transmembrane efficiency of the ESH recombinant protein is time-dependent. The experimental results are as follows: Figure 15 As shown, the intracellular fluorescence intensity increases as the incubation time prolongs, and the fluorescence in HeLa cells can be clearly o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com