Functional peptide fiber, production method thereof and method for recovering peptide chains
a technology peptide chains, which is applied in the field of functional peptide fibers, production methods thereof, and methods for recovering peptide chains, can solve the problems of not knowing a method effective in incorporating functionality into fibers, and it becomes difficult to constitute peptide fibers stably
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0111] (1) Synthesis of Peptides Having .alpha.-Helix Structure
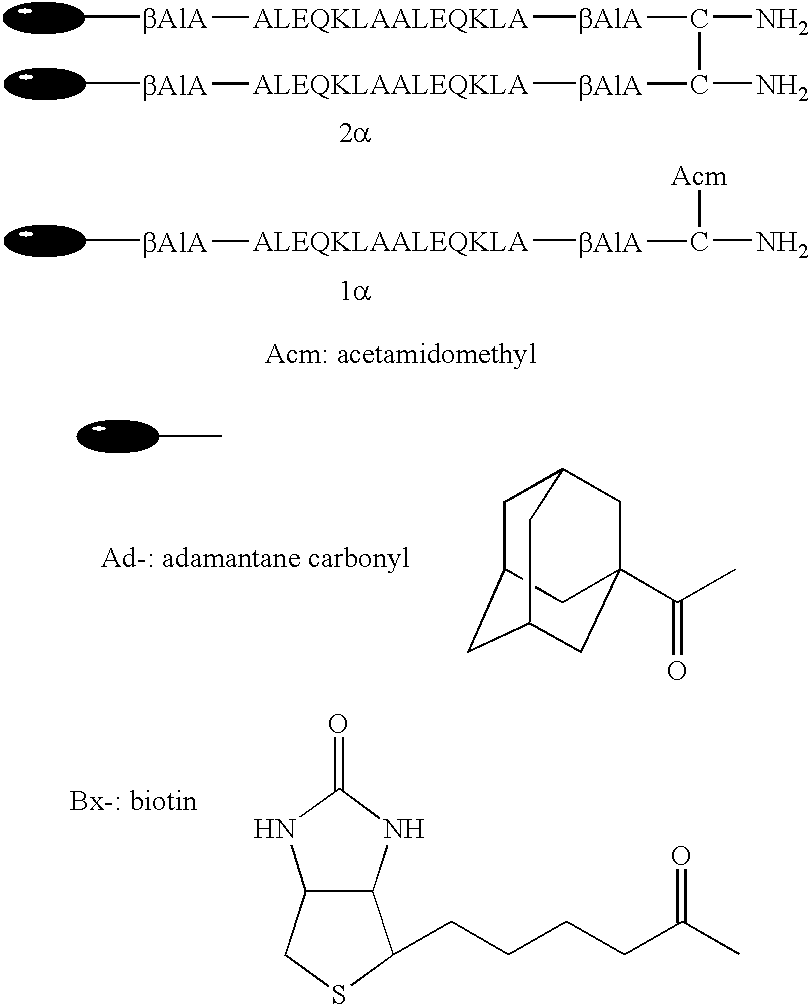
[0112] Peptides shown in the following were designed and synthesized into amphipathic 2.alpha.-helix structure. 1
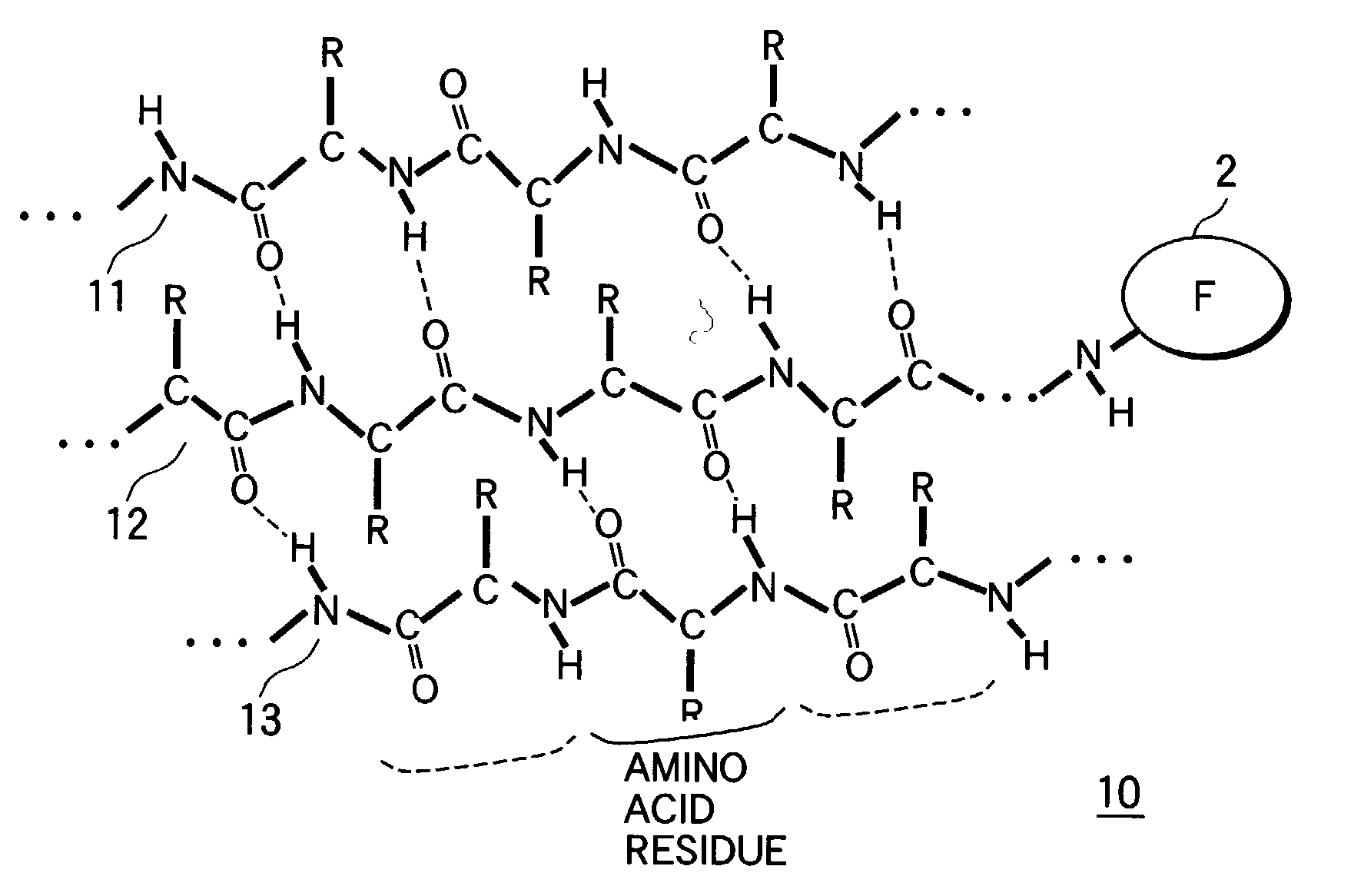
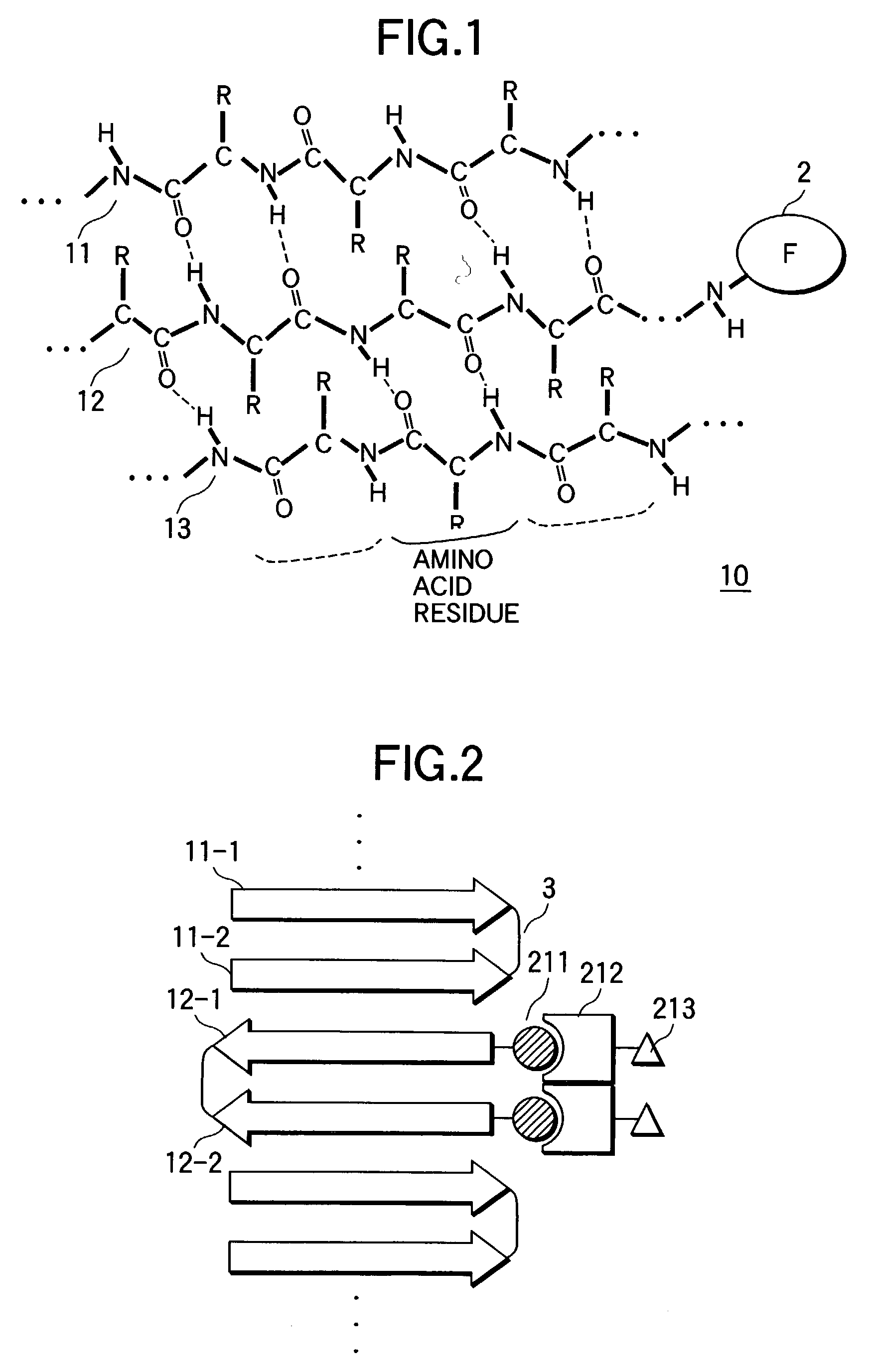
[0113] Adamantane carbonyl group was introduced into the amino terminal as a hydrophobic group to obtain a peptide (Ad-2.alpha.). In the same manner, a peptide (Bx-2.alpha.) whose amino terminal was biotinylated were designed and synthesized. They were formed into a parallel double chain peptide by intermolecular disulfide bond between cysteine residues of their carboxyl terminals. Single chain Ad-1.alpha. and Bx-1.alpha. were also synthesized. Their synthesis was carried out by the general 9-fluorenylmethoxycarbonyl (Fmoc) solid phase method described, e.g., by Takahashi, Y., Ueno, A. and Mihara, H., in Chem., Eur. J., 4, pp. 2475-2484. That is, Ad-1.alpha. and Bx-1.alpha. were synthesized by elongating Fmoc amino acid derivatives (each in 3 to 6 equivalent) corresponding to their amino acid sequences on Rink...
example 2
[0120] A functional fiber in which intervals between functional materials were controlled was obtained by carrying out the same procedure of Example 1, except that both of the peptide (Ad-2.alpha.) in which adamantane carbonyl group was introduced into the amino terminal as a hydrophobic group and the peptide (Bx-2.alpha.) whose amino terminal was biotinylated, obtained in Example 1(1), were used in 1(2) (from 1 to 1,000 .mu.M in concentration, from 1:1 to 100:1 in molar ratio).
example 3
[0121] When a peptide in which its amino terminal was biotinylated and further connected with avidin protein was produced instead of the peptide (Bx-2.alpha.) whose amino terminal was biotinylated in Example 1(1), and it was used in 1(2), it was able to form it into a .beta.-peptide fiber.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com