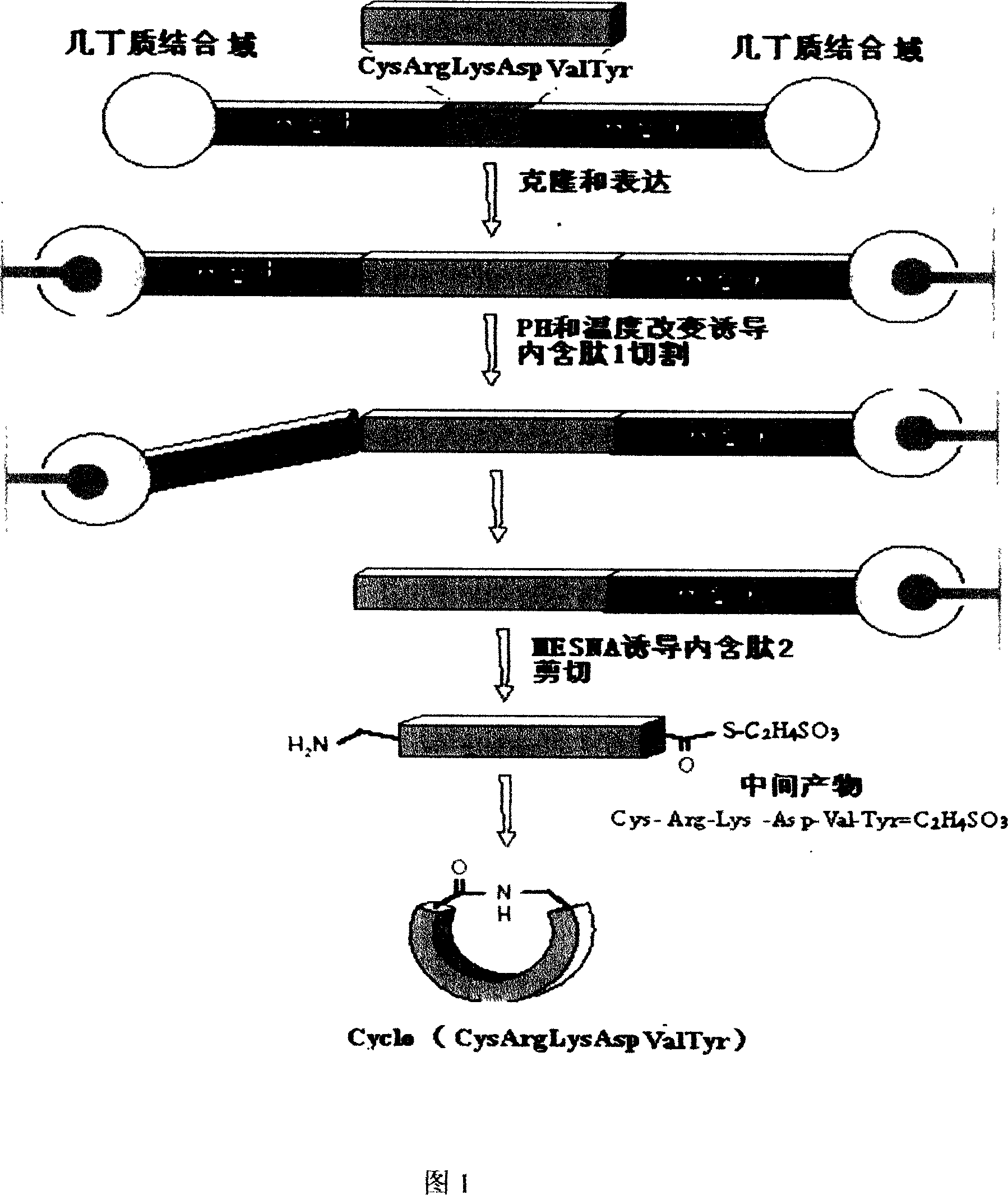
Recombinant thymus pentapeptide structural analogs, its production and use
A technology similar in structure to thymosin, applied in the field of genetic engineering, can solve problems such as the inability to realize the efficient preparation of cyclic peptides, and achieve the effect of low cost and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Expression of recombinant thymopentin structural analog
[0033] 1. Synthesis of recombinant thymosin gene and construction of pTW-TP plasmid
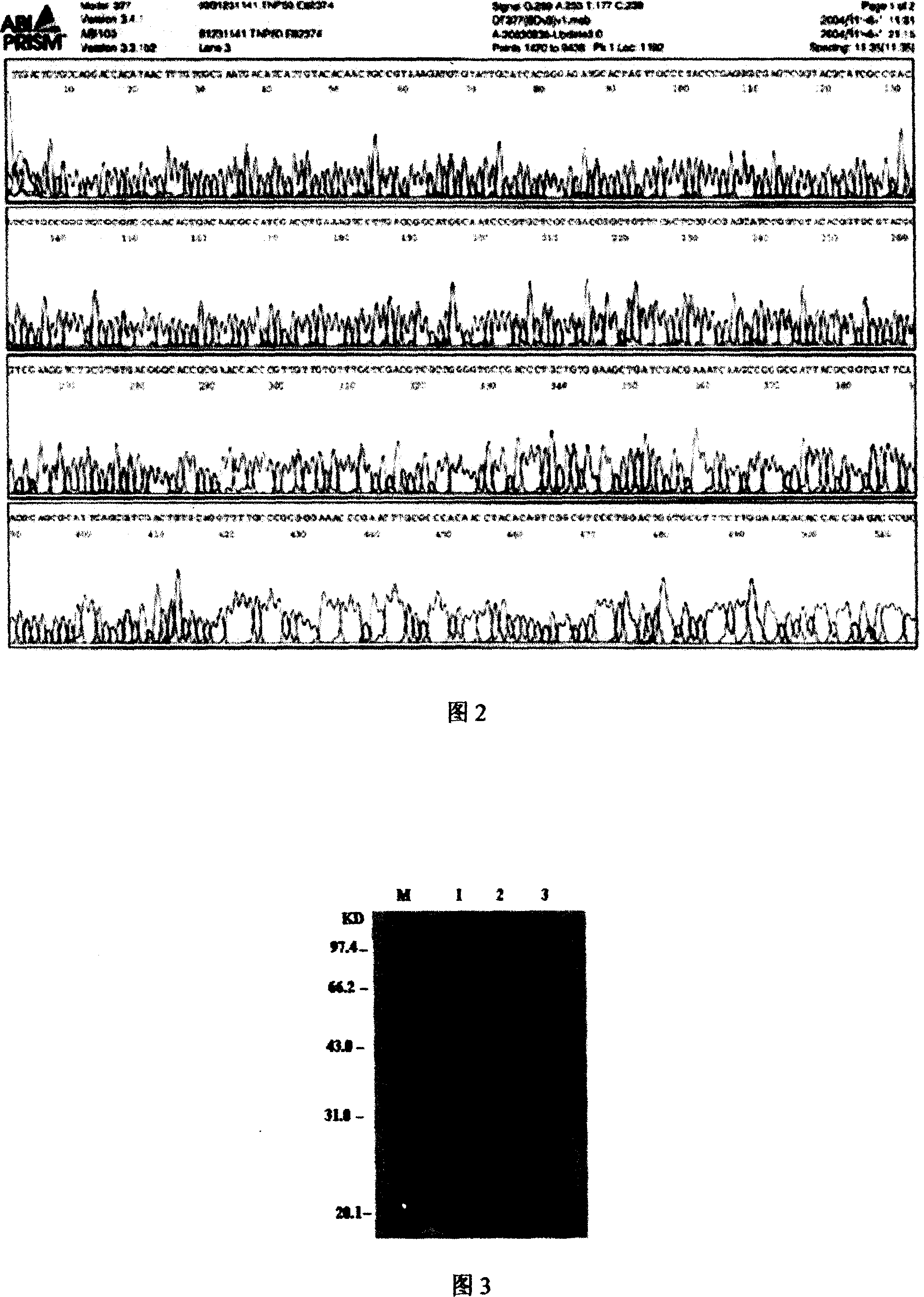
[0034] Two complementary primers were synthesized: primer 1: AAC TGC CGT AAA GAT GTG TAT; primer 2: GCA ATA CAC ATC TTT ACG GCA; the recombinant thymosin gene with sticky ends was artificially synthesized by primer denaturation and renaturation; The cut pTWIN1 vector was ligated to obtain the recombinant expression plasmid pTW-TP. Its sequence diagram is shown in 2.
[0035] 2. Expression and purification of recombinant fusion protein
[0036]The expression plasmid was transformed into the expression host strain E.coli Strain ER2566, and the expression engineered strain pTW-TP-ER2566 was constructed, as shown in Figure 3. Pick the monoclonal shaking bacteria and culture overnight, inoculate 1L LB medium containing 100mg / L ammonium biline at 1:20, shake the bacteria at 37°C until the OD is 0.5-0.8, add IPTG to the...
Embodiment 2
[0039] Example 2 The carbon particle clearance test detects the activity of cyclo in promoting the phagocytosis of macrophages
[0040] cyc-TP and chemically synthesized linear pentapeptide TP-5 (Hainan Zhonghe Pharmaceutical) were intraperitoneally injected into NIH mice (20 g) at a dose of 5 μg / kg, and injected with a blank vehicle as a control, administered once a day for 10 consecutive days . Phagocytosis rate and phagocytosis index were determined and calculated according to literature methods. The results were tested by t.
[0041] The results (as shown in Table 1) showed that the phagocytosis rate (K) and phagocytosis index (a) of cyc-TP and TP-5 were significantly higher than that of the blank solvent control group, and had the effect of significantly improving the clearance ability. Meanwhile, the phagocytic ability of cyc-TP group was higher than that of TP-5 group.
[0042] Table 1: Effect of cyc-TP on washout capacity
[0043] Group
[0044] a: P<0.01...
Embodiment 3
[0045] Example 3 Mouse hemolytic plaque detection activity of cyclo promoting B cell function
[0046] The grouping of the mice, the dosage and the way of administration were the same as before, and the administration was performed once a day for 10 consecutive days, and a blank vehicle was injected as a control. On the 7th day of administration, each mouse was intraperitoneally injected with 0.2ml of 20% SRBC physiological saline mixed solution for immunization. On the second day after the last administration, hemolyzed plaque PFC was measured according to the literature method, and the results (see Table 2 ) through the t-test. The results showed that both cyc-TP and TP-5 effectively promoted the activity and function of B cells, and the activity of cyc-TP was stronger than that of TP-5.
[0047] Table 2: Effect of cyc-TP) on hemolytic plaque formation
[0048] Group
[0049] a: P<0.01, Experimental group vs Control; b: P<0.01, cyc-TP group vs TP-5 group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com