Pentaerythrityl tetramizole and preparation method thereof
A technology of pentatetraimidazole and pentaerythritol, applied in the direction of organic chemistry, etc., can solve problems such as difficulty in synthesizing tetrahedral structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
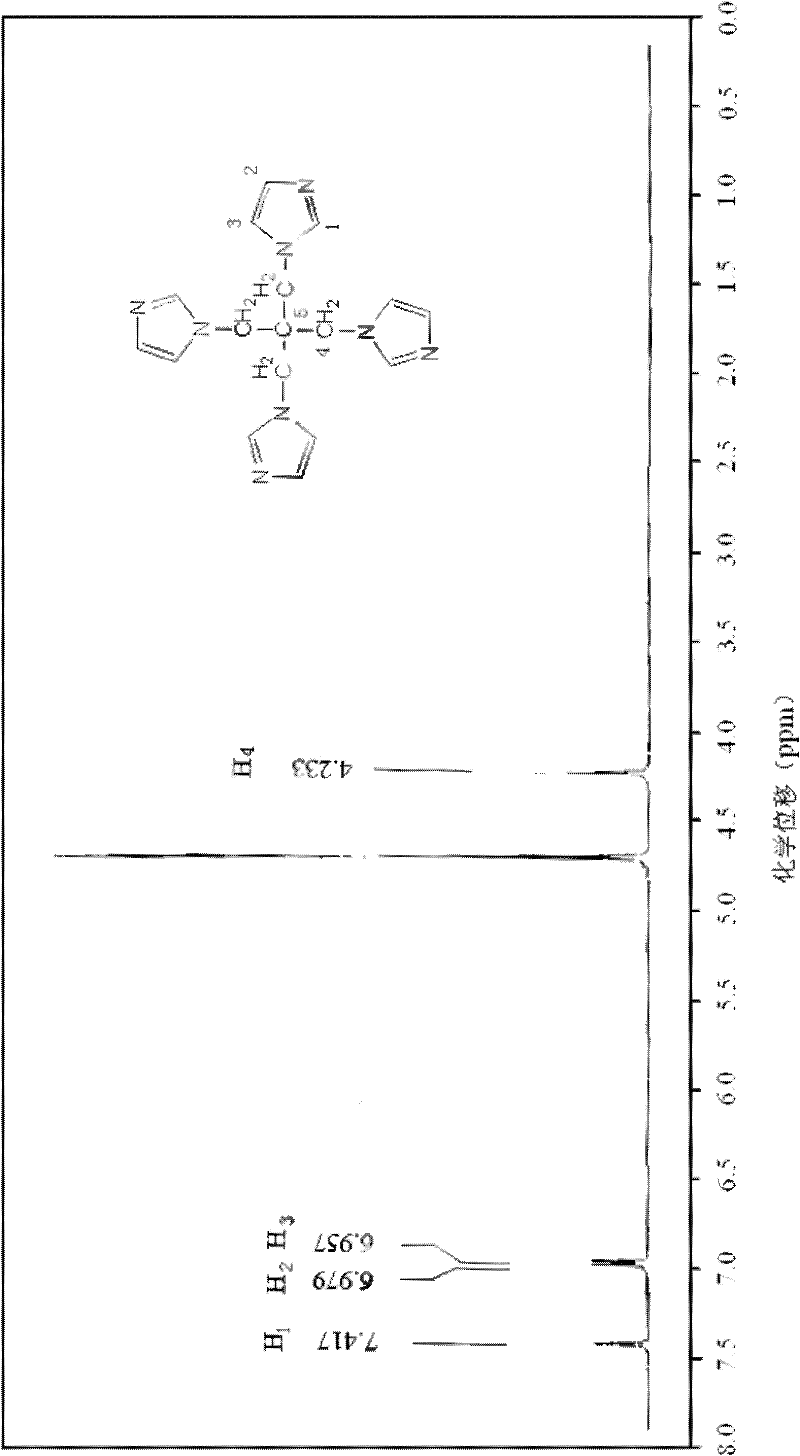
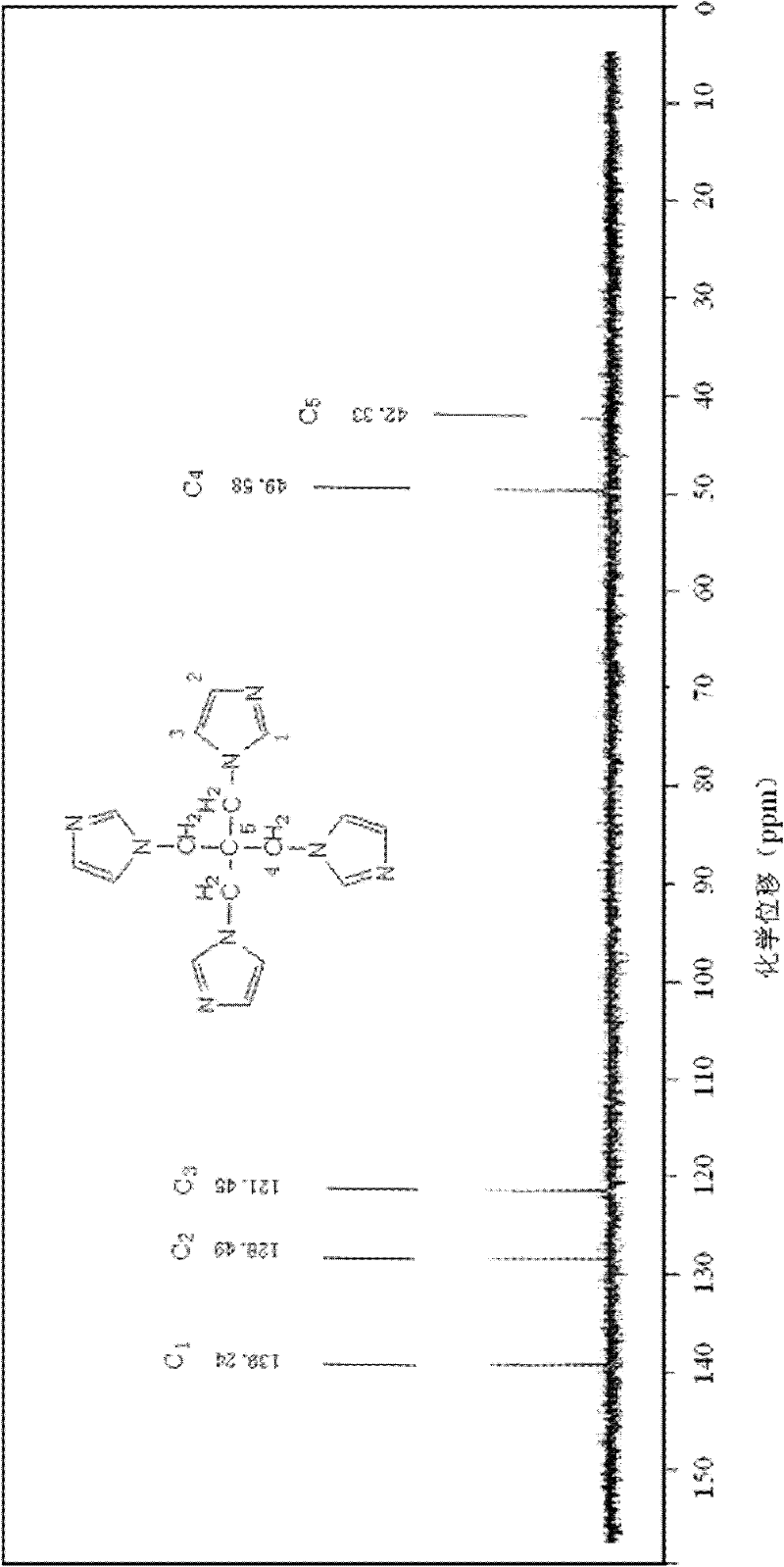
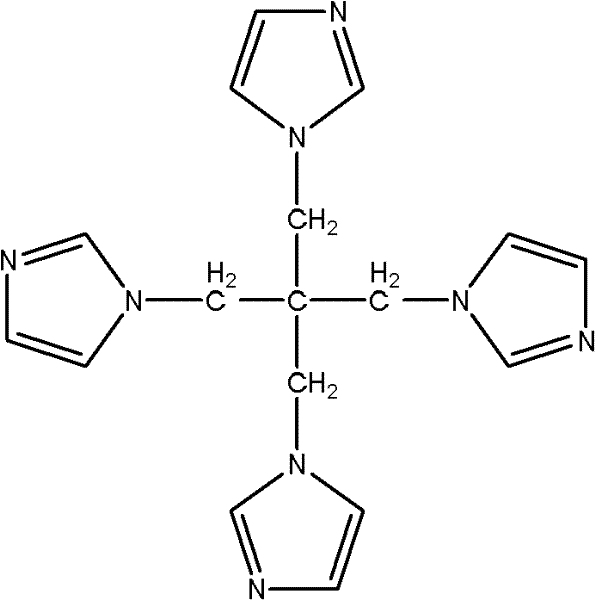
[0016] Specific embodiment 1: The structural formula of pentatetraimidazole in this embodiment is:
[0017]
[0018] The pentaerythritol of this embodiment is a derivative of pentaerythritol. Because of its steric tetrahedron and imidazole cyclic structure, it can be used as an initiator for the synthesis of multi-arm polymers and an important intermediate for the synthesis of dendrimers. Used as a carrier for the synthesis of polyacid catalysts.
specific Embodiment approach 2
[0019] Specific embodiment 2: The preparation method of pentaerythritol of this embodiment is carried out according to the following steps: 1. According to the molar ratio of pentaerythritol and benzenesulfonyl chloride of 1:4 to 4.6, the molar ratio of pentaerythritol to pyridine is 1:5 to Weigh pentaerythritol, benzenesulfonyl chloride and pyridine in a ratio of 7; then measure hydrochloric acid, water and methanol at a ratio of 1:1-2 to 2 by volume of hydrochloric acid and water. Add the pentaerythritol and pyridine weighed in step one to the three-necked flask, and add the benzenesulfonyl chloride weighed in step one dropwise to the three-necked flask under stirring conditions to obtain a mixed solution. In the flask, control the temperature to 25℃~30℃ during the dripping process. After the dripping is completed, heat up to 40℃~42℃ and continue stirring for 2.5h~3h to obtain the mixture; 3. Add the mixture obtained in step 2 to step In the prepared mixed solution, stir it e...
specific Embodiment approach 3
[0020] Specific embodiment three: This embodiment is different from specific embodiment two in that in step one, the molar ratio of pentaerythritol to benzenesulfonyl chloride is 1:4.1-4.5, and the molar ratio of pentaerythritol to pyridine is 1:5.2-6.8. Others are the same as the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com