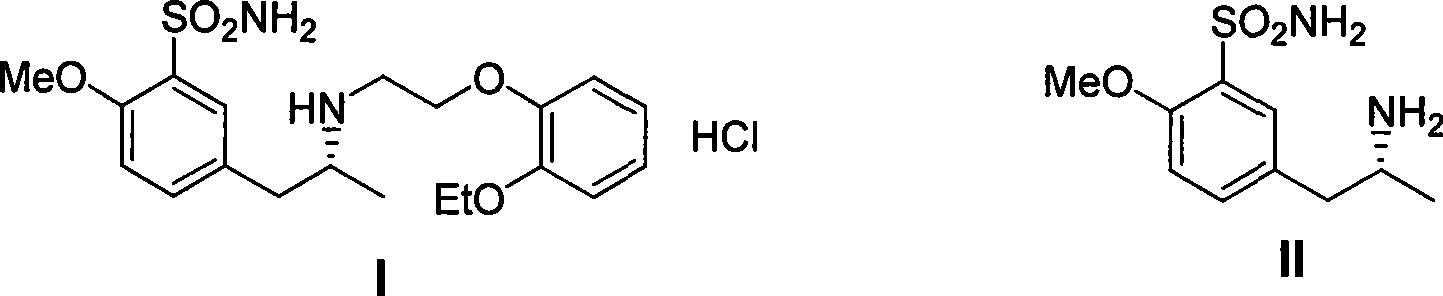
Method for synthesizing (R)-5-(2-amino propyl)-2-methoxybenzenesulfonamide
A kind of technology of methoxybenzenesulfonamide and synthetic method, applied in the synthesis field of -5--2-methoxybenzenesulfonamide, can solve problems such as long route, high price, unavoidable by-products, etc., achieve simplified purification The method, the price is cheap, and the effect is conducive to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
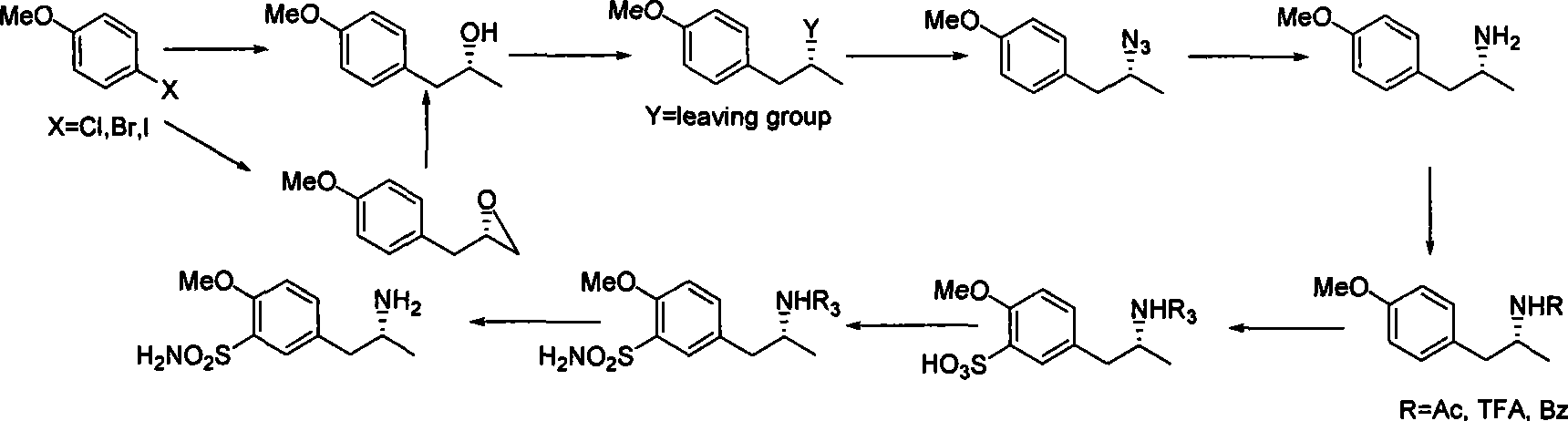
[0032] In a 500ml dry four-necked bottle, add 250ml of anhydrous tetrahydrofuran and 12g of magnesium powder, dropwise add 1ml of dibromoethane, add dropwise 70g of p-methoxychlorobenzene after triggering, control the dropping speed, make the reaction solution slightly reflux, and complete the addition , heating to reflux until the magnesium powder disappears. Cool to 0-5 degrees, add 29g of chiral propylene oxide dropwise, add saturated ammonium chloride aqueous solution to extract the reaction after adding and stir for 2 hours, concentrate THF, extract with ethyl acetate, perform conventional post-treatment, and the obtained product can be used directly react in the next step.
[0033] Dissolve the product from the previous step in 300ml of dichloromethane, add 100ml of triethylamine and 100g of p-toluenesulfonyl chloride, react overnight at room temperature, concentrate, add water and ethyl acetate for extraction, and obtain 120g of a white solid after conventional post-tre...
Embodiment 2
[0038] In a 1000ml dry four-neck flask, add 500ml of anhydrous tetrahydrofuran and 24g of magnesium powder, dropwise add 1ml of dibromoethane, add dropwise 187g of p-methoxybromobenzene after triggering, control the dropping speed, make the reaction solution slightly reflux, and complete the addition , heating to reflux until the magnesium powder disappears. Cool to 0-5 degrees, add 60g of chiral propylene oxide dropwise, add saturated ammonium chloride aqueous solution to extract the reaction after adding and stir for 2 hours, concentrate THF, extract with ethyl acetate, and perform conventional post-treatment. The obtained product can be used directly react in the next step.
[0039] Dissolve the product from the previous step in 600ml of dichloromethane, add 200ml of triethylamine and 200g of p-toluenesulfonyl chloride, react overnight at room temperature, concentrate, add water and ethyl acetate for extraction, and obtain 250g of a white solid after conventional post-treat...
Embodiment 3
[0044] In a 500ml dry four-necked bottle, add 250ml of anhydrous tetrahydrofuran and 12g of magnesium powder, dropwise add 1ml of dibromoethane, add dropwise 70g of p-methoxychlorobenzene after triggering, control the dropping speed, make the reaction solution slightly reflux, and complete the addition , heating to reflux until the magnesium powder disappears. Cool to 0-5 degrees, add 47g of chiral epichlorohydrin dropwise, add saturated ammonium chloride aqueous solution to extract the reaction after adding and stir for 2 hours, concentrate THF, extract with ethyl acetate, and perform conventional post-treatment, the obtained product dissolves In methanol, add 20 g of palladium carbon, hydrogenate at normal pressure, filter after the reaction, concentrate to dryness and directly use in the next reaction.
[0045] Dissolve the product from the previous step in 300ml of dichloromethane, add 100ml of triethylamine and 100g of p-toluenesulfonyl chloride, react overnight at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com