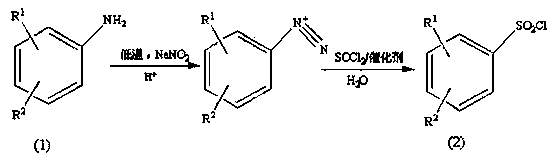
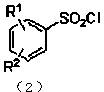
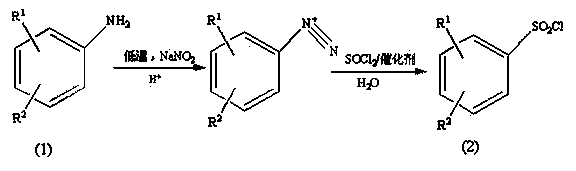
Preparation method of substituted benzene sulfonyl chloride
A technology of benzenesulfonyl chloride and acyl chloride, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of high requirements for reaction equipment, unfavorable industrial production, and long reaction steps, so as to achieve easy control of the reaction and reduce the discharge of "three wastes", equipment less demanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 p-chlorobenzenesulfonyl chloride
[0028] Add 12.7g of p-chloroaniline into a three-necked flask containing 25.4g of 75% sulfuric acid, place the three-necked flask in an ice bath, and slowly add 20mL of sodium nitrite aqueous solution (5.5mol L -1 ), after the dropwise addition was completed, the reaction was stirred for 20 min to obtain a diazonium salt solution.
[0029] Add 23.8g of thionyl chloride into a constant pressure dropping funnel, slowly drop it into a double-necked flask filled with 14.5mL of water under an ice bath, add 2.69g of copper chloride after the drop, and then use glue under ice bath conditions. The diazonium salt solution was quickly added to the aqueous solution with a head dropper, and then stirred at room temperature for 20 min. The pH of the system was adjusted to 2 with saturated potassium carbonate solution, extracted several times with ethyl acetate, and 16.5 g of a light yellow solid was obtained by rota...
Embodiment 2
[0030] Embodiment 2 Preparation of p-chlorobenzenesulfonyl chloride
[0031] Add 12.7g of p-chloroaniline into a three-necked flask filled with 38.1g of concentrated hydrochloric acid, place it in an ice bath, control the temperature at about 0-5°C, and slowly add 20mL of sodium nitrite aqueous solution (6.0mol L -1 ), keep the temperature below 5°C during the dropwise addition, and stir for 60 min to obtain a diazonium salt solution.
[0032] Add 35.7g of thionyl chloride into the constant pressure dropping funnel, slowly drop it into a double-necked flask filled with 29mL of water under an ice bath, add 0.99g of cuprous chloride after dropping, and then use a rubber-tip dropper to drop the heavy Nitrogen salt solution was quickly added to the aqueous solution, and then stirred at room temperature for 20 min, extracted several times with ether, adjusted to pH 4 with saturated potassium carbonate solution, and rotary evaporated to obtain a light yellow solid with a yield of 91...
Embodiment 3
[0033] Example 3 Preparation of 2-nitro-4-chlorobenzenesulfonyl chloride
[0034] Add 17.3g of 2-nitro-4-chloroaniline into a three-necked flask containing 103.8g of concentrated hydrochloric acid, place it in an ice bath, control the temperature at about -5 to 0°C, and slowly add 20mL of sodium nitrite aqueous solution (5.25 mol L -1 ), keep the temperature below 0°C during the dropwise addition, and stir for 20 minutes to obtain a diazonium salt solution.
[0035] Add 59.5g of thionyl chloride into the constant-pressure dropping funnel, slowly drop it into a double-necked flask containing 116mL of water under an ice bath, add 0.8g of cuprous chloride after dropping, and then use a rubber-tip dropper to remove the heavy Nitrogen salt solution was quickly added to the aqueous solution, and after completion, stirred at room temperature for 10 min, extracted several times with dichloromethane, adjusted the pH to 8 with saturated potassium carbonate solution, and rotary evapor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com