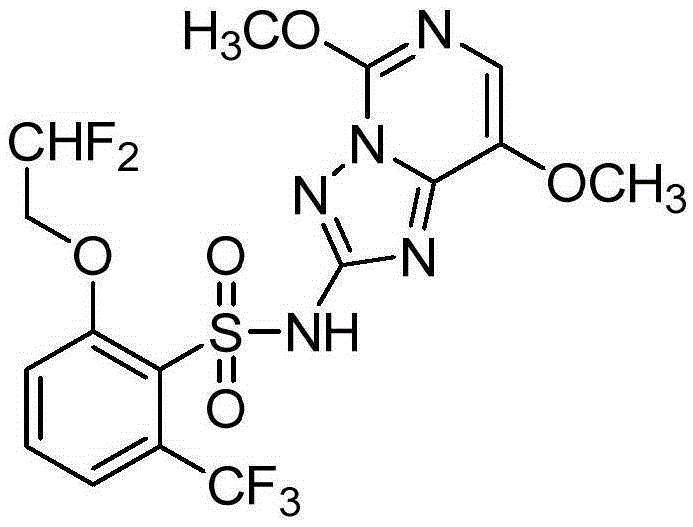
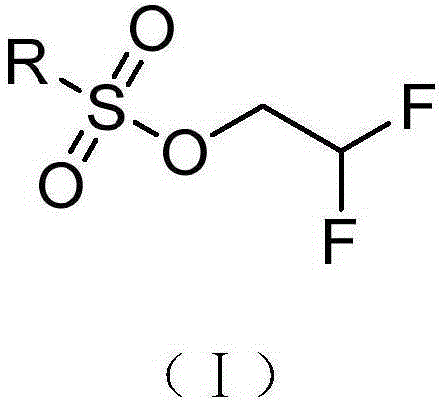
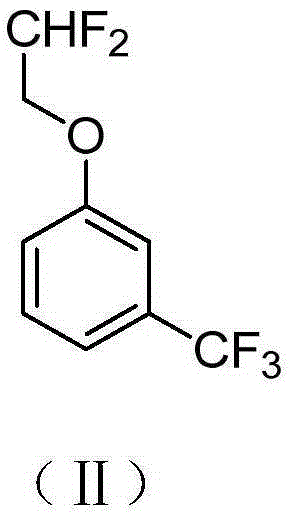
Preparation method of 2-(2',2'-difluoroethoxy)-6-(trifluoromethyl)benzene-1-sulfonyl chloride
A technology of trifluoromethylbenzenesulfonyl chloride and difluoroethoxy is applied in the field of preparation of 2--6-trifluoromethylbenzenesulfonyl chloride, and can solve the problems of high price, reduced yield, potential safety hazards and the like, To achieve the effect of simple operation and improve synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of 2-(2',2'-difluoroethoxy)-6-trifluoromethylbenzenesulfonyl chloride, comprising the steps of:
[0035]
[0036] (1) 100 g of 2,2-difluoroethanol, 160 g of triethylamine and 900 mL of anhydrous dichloromethane were added to a 2 L round bottom flask. At 0°C, 146.6 g of methanesulfonyl chloride was slowly added dropwise to the above solution, and after naturally rising to room temperature, the reaction was carried out for 4 hours. The reaction solution was poured into ice water, the organic phase was separated, washed with water, washed with salt water, dried, and spin-dried to obtain a crude product. After rectification, compound (Ⅲ) (173.9 g, light yellow liquid) was obtained, with a yield of 89%. Proton NMR spectrum (400MHz, CDCl 3 ) δ: 3.13(s, 3H), 4.39(dt, J=3Hz, J=13Hz, 2H), 6.02(tt, J=3Hz, J=55Hz, 1H).
[0037]
[0038] (2) Add 160g of compound (Ⅲ), 162g of m-trifluoromethylphenol and 207g of anhydrous potassium carbonate into 1000mL o...
Embodiment 2
[0042] A preparation method of 2-(2',2'-difluoroethoxy)-6-trifluoromethylbenzenesulfonyl chloride, comprising the steps of:
[0043]
[0044] (1) 100 g of 2,2-difluoroethanol, 205 g of triethylamine and 900 mL of anhydrous dichloromethane were added to a 2 L round bottom flask. At 0°C, 200 g of trifluoromethanesulfonic anhydride was slowly added dropwise to the above solution, and after naturally rising to room temperature, the reaction was carried out for 5.2 hours. The reaction solution was poured into ice water, the organic phase was separated, washed with water, washed with brine, dried, and spin-dried to obtain a crude product. After rectification, compound (IV) (120.1 g, light yellow liquid) was obtained, with a yield of 61%. Proton NMR spectrum (400MHz, CDCl 3 ) δ: 4.58 (dt, J=3.6, 12.8Hz, 2H), 6.05 (tt, J=3.6, 54Hz, 1H).
[0045]
[0046] (2) Under ice bath conditions, 160 g of compound (IV), 90.8 g of m-trifluoromethylphenol and 55 g of anhydrous pyridine were a...
Embodiment 3
[0050] A preparation method of 2-(2',2'-difluoroethoxy)-6-trifluoromethylbenzenesulfonyl chloride, comprising the steps of:
[0051]
[0052] (1) 100 g of 2,2-difluoroethanol, 160 g of triethylamine and 900 mL of anhydrous dichloromethane were added to a 2 L round bottom flask. At 0°C, 146.6 g of methanesulfonyl chloride was slowly added dropwise to the above solution, and after naturally rising to room temperature, the reaction was carried out for 4 hours. The reaction solution was poured into ice water, the organic phase was separated, washed with water, washed with salt water, dried, and spin-dried to obtain a crude product. After rectification, compound (Ⅲ) (173.9 g, light yellow liquid) was obtained, with a yield of 89%. Proton NMR spectrum (400MHz, CDCl 3 ) δ: 3.13(s, 3H), 4.39(dt, J=3Hz, J=13Hz, 2H), 6.02(tt, J=3Hz, J=55Hz, 1H).
[0053]
[0054] (2) 160 g of compound (III), 162 g of m-trifluoromethylphenol and 207 g of anhydrous potassium carbonate were added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com