Preparation method of 2-(2,2-difluoroethyoxyl)-6-trifluoromethyl benzenesulfonyl chloride
A technology of trifluoromethylbenzenesulfonyl chloride and difluoroethoxy, which is applied in the field of preparation of 2--6-trifluoromethylbenzenesulfonyl chloride, can solve environmental and production personnel injuries, high safety hazards, chlorine gas, and cost High-level problems, to achieve the effect of eliminating malodorous odor, high purity and yield, and improving production safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
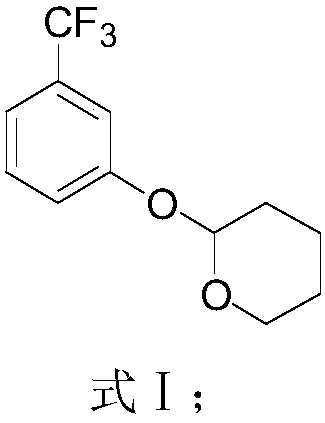
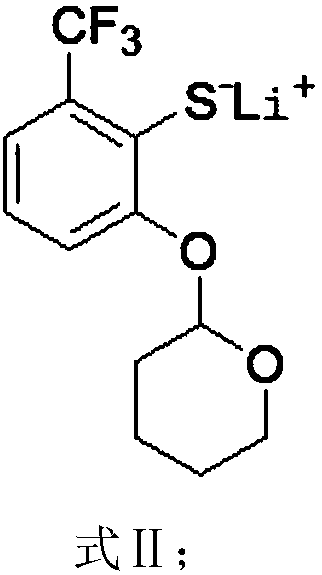
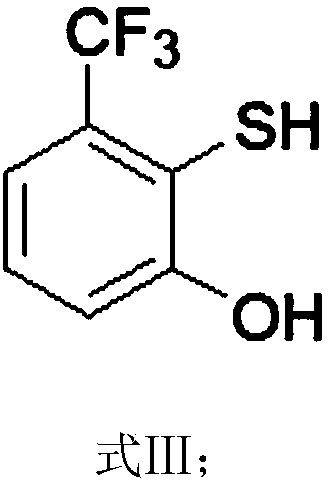
[0066] The synthesis route of 2-(2,2-difluoroethoxy)-6-trifluoromethylbenzenesulfonyl chloride in this example is as follows, and the specific preparation method includes the following steps:
[0067]
[0068] 1) Add m-trifluoromethylphenol 40.0g (246mmol), dichloromethane 160ml, p-toluenesulfonic acid 0.2g (1.24mmol) into the reaction flask, add 24g 3,4-dihydropyran under cooling to 0°C (295.2mmol), react at 25°C for 2h, add triethylamine to adjust the pH to neutral after the reaction, add 80ml of water after stirring for 10 minutes, let stand to separate layers, add dichloromethane (20ml*3) to extract the water layer , washed, dried over anhydrous magnesium sulfate, and the dichloromethane layer was recovered to obtain a pale yellow liquid, namely the compound represented by formula I (59.9 g, yield 99.0%). 1 H-NMR (300MHZ, CDCl3) δ7.41-7.21 (m, 4H, Ar-H), 5.46 (t, J = 3.1Hz, 1H, pyran ring hydrogen), 3.92-3.84 (m, 1H, pyran ring hydrogen), 3.66-3.60 (m, 1H, pyran ring h...
Embodiment 2
[0076] The preparation method of the present embodiment 2-(2,2-difluoroethoxy)-6-trifluoromethylbenzenesulfonyl chloride comprises the following steps:
[0077] 1) Add m-trifluoromethylphenol 32.0g (197.4mmol), 150ml tetrahydrofuran, ammonium chloride 0.21g (3.94mmol) into the reaction flask, add 3,4-dihydropyran 17.4g (207.3mmol) at room temperature , after the dropwise addition, heat up and react at 66°C for 1 hour. After the reaction, cool down to room temperature and add triethylamine to adjust the pH to neutral. After stirring for 10 minutes, evaporate tetrahydrofuran under reduced pressure, add 65ml of water, and dichloro Extracted with methane (60ml×3), washed the dichloromethane layer with water, dried over anhydrous magnesium sulfate, recovered the dichloromethane to obtain a light yellow liquid, namely the compound shown in formula I (47.1g, content 97.6%, yield 97.0%) ;
[0078] 2) Add 24g (97.2mmol) of the compound shown in Formula I, 0.574g (9.72mmol) of isopropy...
Embodiment 3
[0084] 1) Add m-trifluoromethylphenol 50.0g (308.4mmol), 500ml acetonitrile, aluminum phosphate 1.88g (15.4mmol) in the reaction flask, add 3,4-dihydropyran 129.5g (1.54mol) at room temperature, After the dropwise addition, raise the temperature to 80°C for reflux reaction for 1 hour. After the reaction, cool down to room temperature and add triethylamine to adjust the pH to neutral. After stirring for 10 minutes, evaporate acetonitrile under reduced pressure, add 100ml of water, and dichloromethane (150ml×3) extracted, washed the dichloromethane layer with water, dried over anhydrous magnesium sulfate, recovered the dichloromethane to obtain a light yellow liquid, namely the compound shown in formula I (74.4g, content 93.6%, yield 98.0%);
[0085] 2) Add 30.0g (121.8mmol) of the compound shown in Formula I, 12.3g (121.8mmol) of triethylamine, 110ml of dry cyclohexane into the reaction flask, cool down to 0°C under the protection of argon, and dropwise add 110.7 The cyclohexan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com