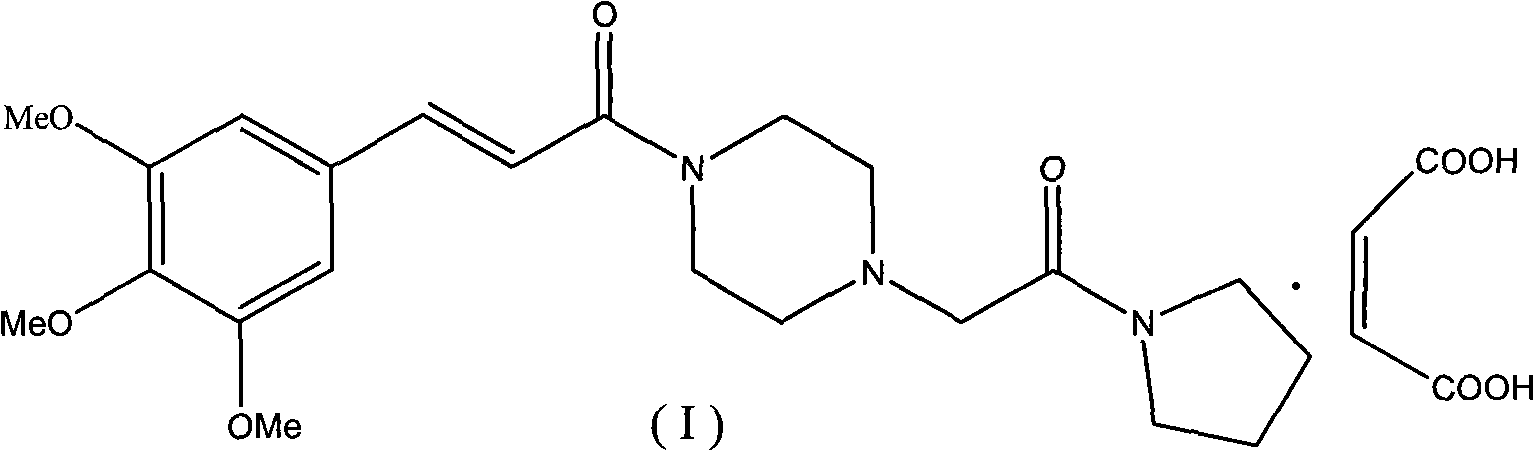
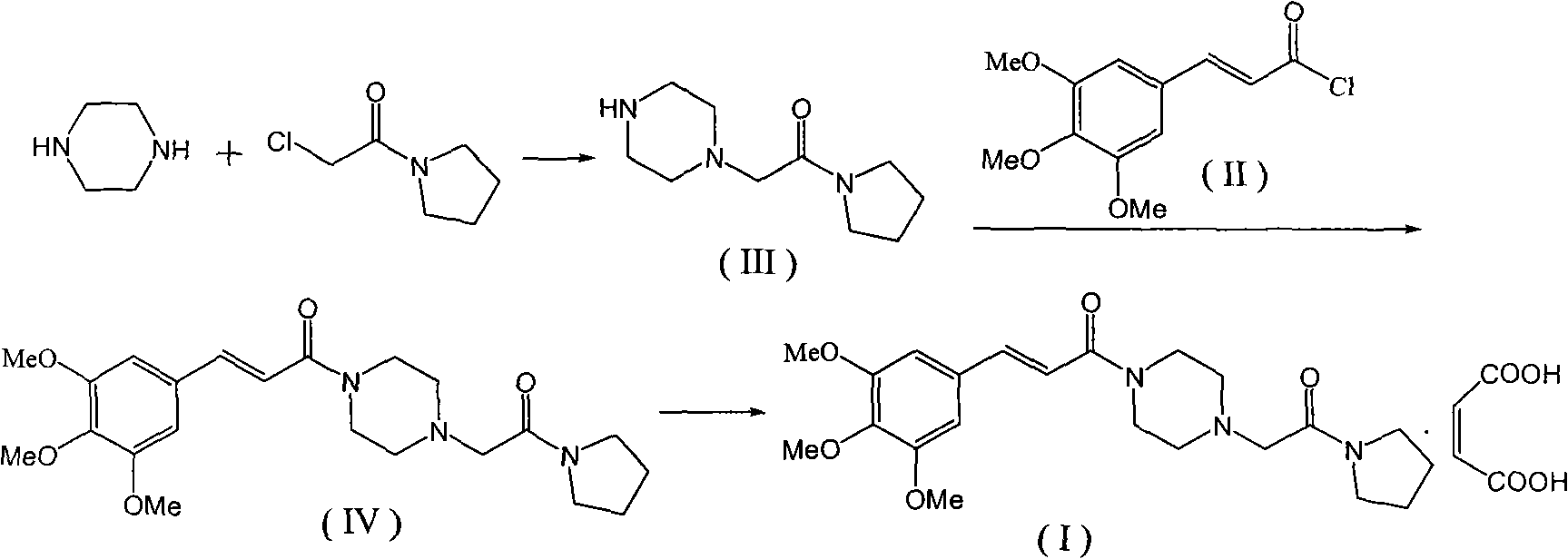
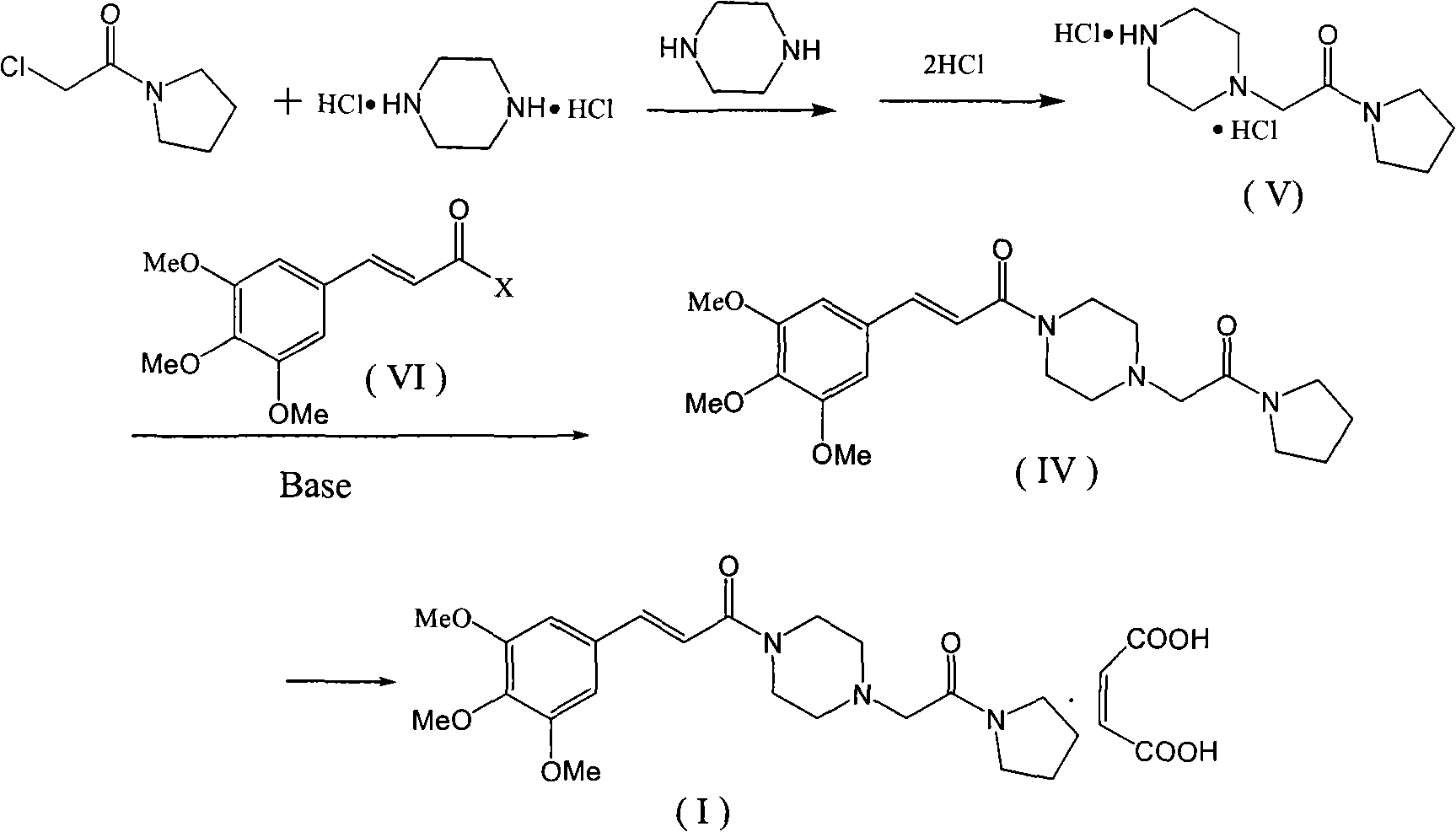
Method for preparing cinepazide maleate
A technology of cinepazide maleate and piperazine, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of compound III extraction and crystallization difficulties, large quantities, etc., and achieve the effect of good crystal form, simple operation process and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1.1, the preparation of chloroacetylpyrrolidine
[0038] Add 22.6g (0.2moL) of chloroacetyl chloride into 70mL of dichloromethane, cool to -10°C; add dropwise 14.21g (0.2moL) of tetrahydropyrrole, 22.26g (0.22moL) of triethylamine in 20mL of di Chloromethane solution, the temperature is controlled below -5°C, after the addition is complete, the reaction is continued at room temperature for 1 h. Washed with 30mL water three times, dried over anhydrous sodium sulfate, filtered, concentrated to near dryness, and left at room temperature to obtain 25.51g of the product with a yield of 86.45%.
[0039] 1.2, Preparation of 1-[(1-pyrrolidinecarbonyl)methyl]piperazine dihydrochloride (V)
[0040] Add 31.8g (0.2mol) of piperazine dihydrochloride and 38.8g (0.2mol) of piperazine hexahydrate into 100mL of absolute ethanol, heat to reflux until fully dissolved. Under stirring, a 70 mL ethanol solution of 29.51 g (0.2 mol) of chloroacetylpyrrolidine was added dropwise, and the dro...
Embodiment 2
[0052] 2.1 Preparation of 3,4,5-trimethoxycinnamic phosphoric anhydride
[0053] Suspend 7.14g (0.03mol) of 3,4,5-trimethoxycinnamic acid in 70mL of dichloromethane solution, stir for 10min, and add 9.12mL (0.066mol) of triethylamine. Cool down to 10°C and add dropwise 4.78mL (0.033mol) of diethyl chlorophosphate in 30mL of dichloromethane solution within 0.5h under stirring, and continue to stir for 1h after the dropwise reaction. spare. or drip
[0054] 2.2. Preparation of 1-[(1-pyrrolidinecarbonyl)methyl]piperazine in dichloromethane
[0055] Suspend 8.1g (0.03moL) of 1-[(1-pyrrolidinecarbonyl)methyl]piperazine dihydrochloride in 20mL of dichloromethane, add 10.1g (0.1mol) of triethylamine, stir at room temperature for 1h, and filter A solid was obtained; the solid was washed with 10 mL of dichloromethane, and combined with the filtrate to obtain a dichloromethane solution of 1-[(1-pyrrolidinecarbonyl)methyl]piperazine.
[0056] 2.3, the preparation of cinepazide maleat...
Embodiment 3
[0060] 3.1 Preparation of 3,4,5-trimethoxycinnamic sulfonic anhydride
[0061] Suspend 11.91g (0.05mol) of 3,4,5-trimethoxycinnamic acid in 10mL of ethyl acetate, and dropwise add 7.6g of triethylamine under stirring to obtain a transparent solution; cool down to -10°C, and dropwise add 10.6g (0.06mol) 20 mL of ethyl acetate solution of benzenesulfonyl chloride, stirred at 0°C for 2 hours, and stirred at room temperature for 1 hour; filtered to obtain crude 3,4,5-trimethoxycinnamic acid benzenesulfonic anhydride.
[0062] 3.2, the preparation of cinepazide maleate
[0063] Suspend 13.5g (0.05mol) of 1-[(1-pyrrolidinecarbonyl)methyl]piperazine dihydrochloride (V) in 200mL of dichloromethane, and add 20.2g (0.2mol) of triethylamine dropwise at room temperature , Continue stirring for 0.5h after addition. Within 1 h, the crude 3,4,5-trimethoxycinnamic acid benzenesulfonic anhydride prepared in Step 3.1 was added in 3 times, and stirred at room temperature for 3 h. The reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com