Industrialization production technology for tenofovir disoproxil fumarate
A production process and reaction technology, which is applied in the field of tenofovir preparation process, can solve the problems of harsh production conditions, high cost, and low yield of large-scale preparation, and achieve the effects of cost reduction, high yield, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
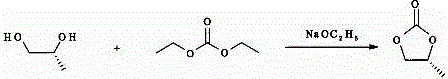
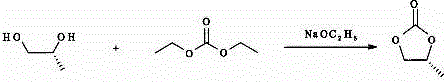
[0018] (1) Preparation of R-propylene carbonate, add (R-1,2-propylene glycol), diethyl carbonate and sodium ethoxide to a glass-lined reactor, heat to 105-110°C, react for 7-9 hours, and react After termination, the unreacted diethyl carbonate is distilled off under reduced pressure, cooled to room temperature, insoluble matter is filtered off, and the filtrate is evaporated to remove the solvent to obtain the product R-propylene carbonate.
[0019] The chemical formula of the above synthesis reaction is:
[0020]
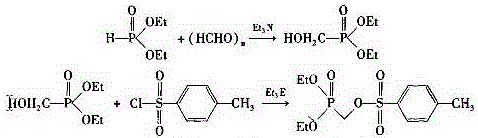
[0021] (2) Preparation of diethyl p-toluenesulfonyloxymethylphosphonate
[0022] Add absolute ethanol and diethyl phosphite to the glass-lined reaction kettle, stir, and the stirring is complete, open the lid of the kettle, put in paraformaldehyde and triethylamine, heat to 35-45°C and react for 0.5-1 hour, at 50- Incubate the reaction at 65°C for 4-6 hours, cool slightly to 30-40°C, filter the solid with diatomaceous earth, and rinse with a small amount of ethanol, comb...
specific Embodiment
[0029] The specific embodiment of the present invention is carried out according to the following steps:
[0030] (1) Preparation of R-propylene carbonate, add R-1,2-propylene glycol (38.05kg, 500mol), diethyl carbonate (70.88kg, 600 mol) and (3.4kg, 50mol) into the glass-lined reactor Sodium ethoxide, heated to 105-110°C, reacted for 8 hours, the reaction was terminated, the unreacted diethyl carbonate was distilled off under reduced pressure, cooled to room temperature, the insoluble matter was filtered off, the filtrate was evaporated to remove the solvent to obtain the product R-carbonic acid The propylene ester is 34.24kg, the purity is ≥99%, and the yield is 90%.
[0031] The chemical formula of the above synthesis reaction is:
[0032]
[0033] (2) Preparation of diethyl p-toluenesulfonyloxymethylphosphonate
[0034] Add absolute ethanol (51 kg, 1107 mol) and diethyl phosphite (22 kg, 159.3 mol) into the glass-lined reactor, stir, and after the stirring is complete, open the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com