Method for synthesizing glufosinate ammonium salt
A synthesis method, the technology of phosphonium ammonium salt, applied in the field of synthesis of glufosinate ammonium salt, can solve the problems of unstable control of the intermediate process, cumbersome separation and purification process, and very serious equipment corrosion, and achieve cost economy and mild reaction conditions Ease of control and high recycling rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
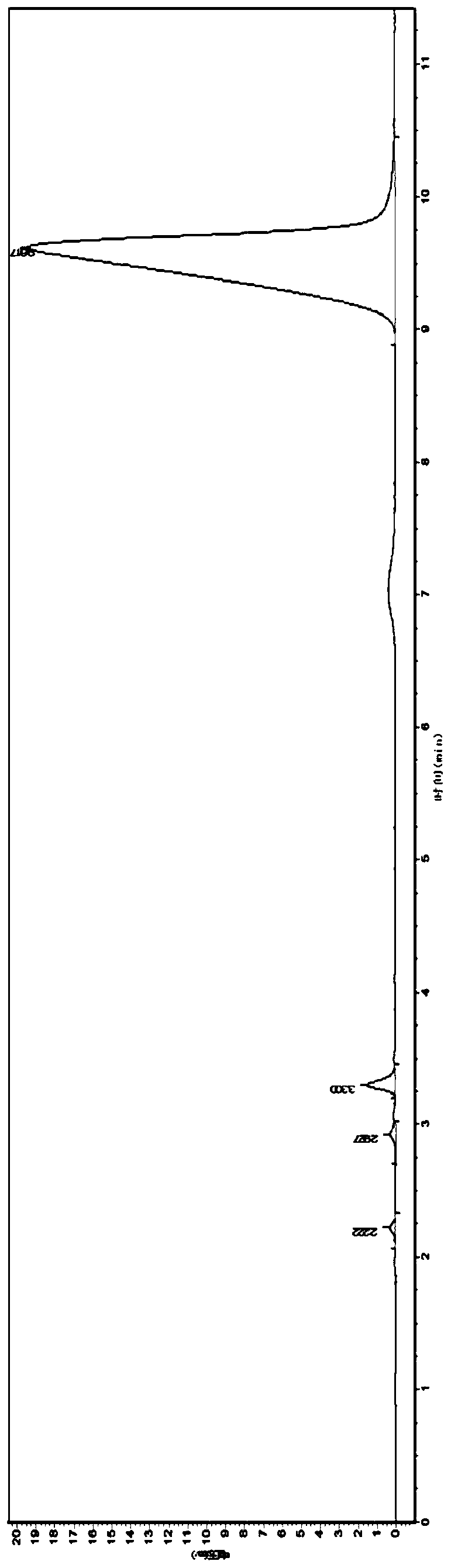
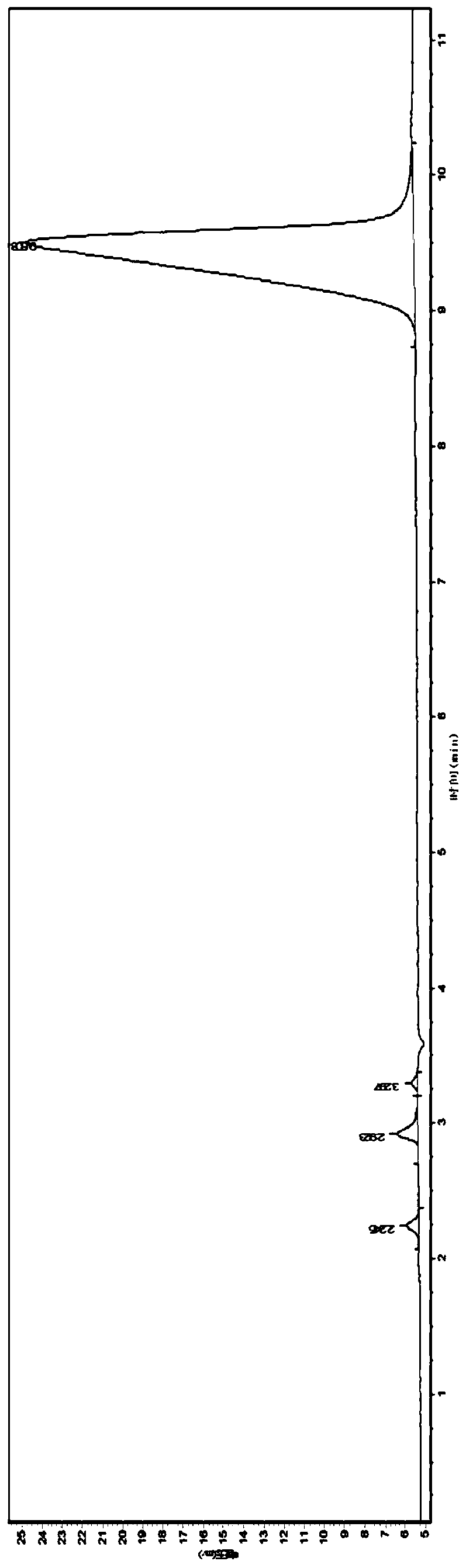
Image
Examples
preparation example Construction
[0036] The synthetic method of glufosinate-ammonium ammonium salt, comprises the following steps,
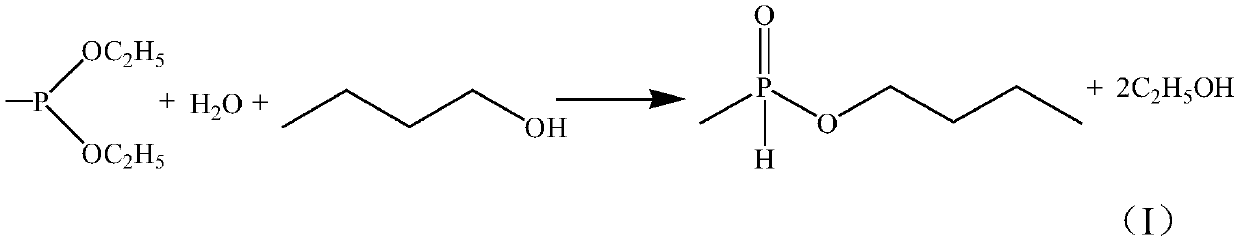
[0037] Step 1. Using diethyl methyl phosphite as a raw material, first react with water, hydrolyze to ethyl methyl hydroxy phosphite or rearrange to ethyl methyl phosphite, and then transesterify with n-butanol in the presence of an initiator Reaction and rearrangement generate monobutyl methyl hypophosphite (I);
[0038] Step 2, methyl hypophosphite monobutyl (I) and acrolein cyanohydrin acetate carry out free radical addition reaction, generate (3-acetoxy group-3-cyanopropyl group) methyl hypophosphite n-butyl ester ( II);
[0039] Step 3, (3-acetoxy-3-cyanopropyl) methyl n-butyl hypophosphite (II) carries out ammonolysis reaction with ammonia gas (liquid ammonia) under 0.0-0.4MPa pressure to generate 3-( Amino-3-cyanopropyl)methyl-n-butylphosphinate (III) or 3-(amino-3-cyanopropyl)methylammonium phosphonate (IV);
[0040] Step 4, 3-(amino-3-cyanopropyl) methyl hypophosphit...
Embodiment 1
[0104] Step 1, prepare monobutyl methyl hypophosphite (I):
[0105] Under nitrogen protection, add 139.0 grams of diethyl methyl phosphite (1.0 mol, gas chromatography purity 98.0%) to a 1.5L dry reaction flask (equipped with a 400mm long rectification column), then add 188 grams of anhydrous n-butanol (2.5mol, purity 99%), then control the temperature of the material at 45°C-50°C, slowly add 18.5 grams (1.02mol) of pure water dropwise, after the dropwise addition, keep warm for 1.0 hour, add 500 ml of toluene, heat up and reflux for dehydration and low temperature Boiling matter; after reflux for about 4 hours, the conversion rate was detected; after detection, the hydrolysis was complete;
[0106] Add 188 gram n-butanols (2.5mol, purity 99%) and 1.0 gram (0.003mol) tetra-n-butyl titanate in reactor again, be warmed up to reflux temperature then, continue to distill out azeotropic low boiler, temperature Gradually increase until the top temperature is 115°C and the low tempe...
Embodiment 2
[0124] Step 1, prepare monobutyl methyl hypophosphite (I):
[0125] Under nitrogen protection, add 139.0 grams of diethyl methyl phosphite (1.0mol, gas chromatography purity 98.0%) to a 1.5L dry reaction flask (equipped with a 400mm long rectification column), then add 267 grams of anhydrous n-butanol (3.6mol, purity 99%), then control the temperature of the material at 45°C-50°C, slowly add 18.9 grams (1.05mol) of pure water dropwise, after the dropwise addition, keep warm for 1.0 hour, add 500 ml of toluene, heat up and reflux for dehydration and low temperature Boiling matter; after reflux for about 4 hours, the conversion rate was detected; after detection, the hydrolysis was complete;
[0126] Add 178 grams of n-butanol (2.4mol, purity 99%) and 0.70 grams (0.002mol) tetra-n-butyl titanate in the reactor again, then be warming up to reflux temperature, continue to distill out azeotropic low boiler, temperature Gradually increase until the top temperature is 115°C and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com