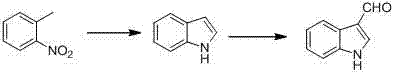
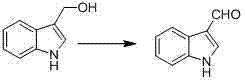
Synthetic method for indole-3-carboxaldehyde compounds
A technology of indole formaldehyde and synthesis method, applied in the direction of organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add o-methylaniline (10 g, 93 mmol) and 10 ml of DMF into the flask, and slowly add 20 ml of Vilsmeier reagent prepared dropwise at 0°C. After the dropwise addition, stir at room temperature (about 25°C) for 1 hour. Then the temperature was raised to 85° C., and the reaction was heated for 5 hours. After the completion of the detection reaction, a saturated sodium carbonate solution was added until it was alkaline, and a large amount of solids were precipitated, filtered, and dried to obtain 12.4 grams of solids, with a yield of 96%. The resulting product had a melting point of 198-199°C.
[0035] 1 H NMR (DMSO-d 6 ) 12.14 (1H, broad), 9.95 (1H, s), 8.30-8.09 (2H, m), 7.56-7.20 (3H, m); 13 C NMR (DMSO-d 6 ) 185.34, 138.85, 137.43, 124.49, 123.84, 122.50, 121.20, 118.54, 112.80.
[0036] Example 2
Embodiment 2
[0038] 4-Amino-3-methylphenol (15 g, 121.8 mmol) and 10 ml of DMF were added to the flask, and 20 ml of the prepared Vilsmeier reagent was slowly added dropwise at 0°C. After the dropwise addition was complete, stir at room temperature for 1 hour. Then the temperature was raised to 85° C., and the reaction was heated for 7 hours. After completion of the reaction, saturated sodium carbonate solution was added until it was alkaline, and a large amount of solids were precipitated. After filtration and drying, 18 grams of solids were obtained, with a yield of 92%. The resulting product had a melting point of 235°C.
[0039] 1 H NMR (DMSO-d 6 ) δ: 11.89 (1H, s), 9.84 (1H, s), 9.07 (1H, s), 8.13 (1H, s), 7.48 (1H, d), 7.29 (1H, d), 6.73 (1H, dd ).
[0040] Example 3
Embodiment 3
[0042] 2,3-Dimethyl-aniline (10 g, 82.5 mmol) and 10 ml of DMF were added to the flask, and 25 ml of the prepared Vilsmeier reagent was slowly added dropwise at 0°C. After the dropwise addition was complete, stir at room temperature for 1 hour. Then the temperature was raised to 85° C., and the reaction was heated for 7 hours. After the reaction was complete, saturated sodium carbonate solution was added until it was alkaline, and a large amount of light yellow solids were precipitated, which were filtered and dried to obtain 11.8 g of solids, with a yield of 90%. The resulting product had a melting point of 198-199°C.
[0043] 1 H NMR (DMSO-d6, 400 MHz) δ: 12.51(1H, broad), 9.97 (1H, s), 7.34 (1H, d), 7.20 (1H, d), 6.98 (1H, d), 6.73 (1H , d), 2.35 (3H, s).
[0044] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com