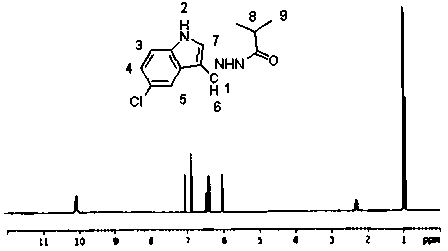
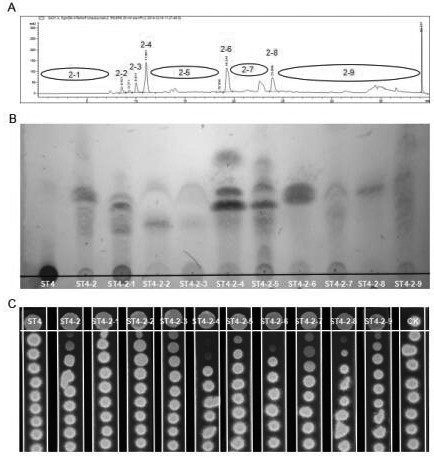
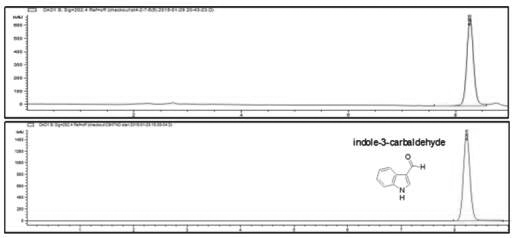
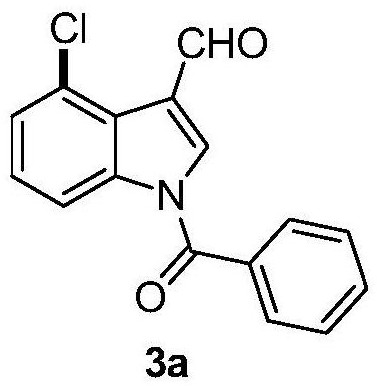
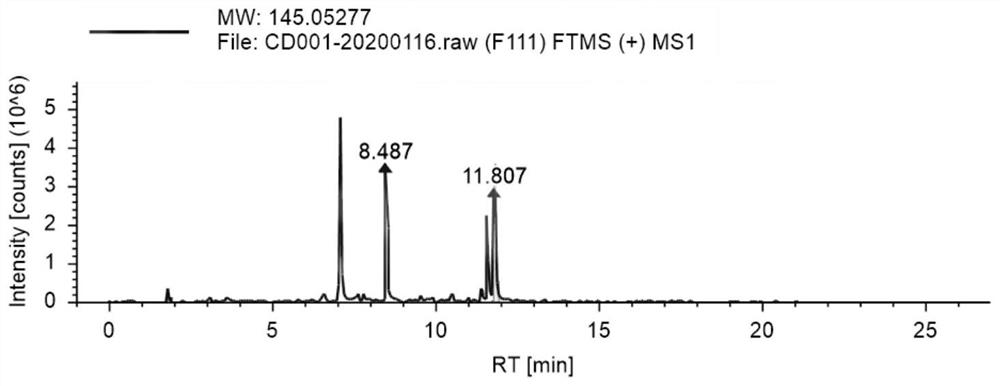
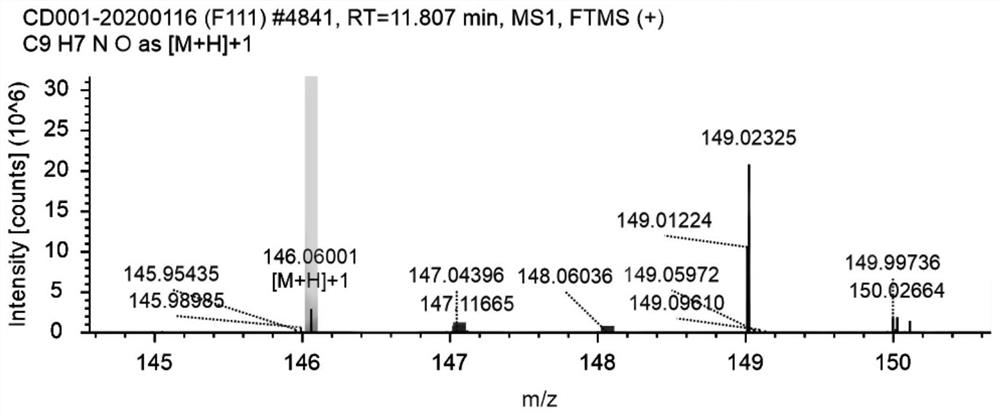
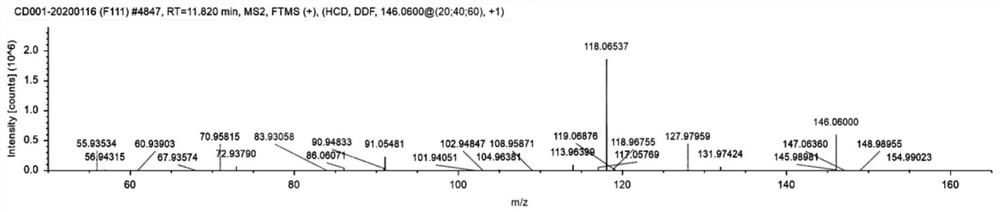
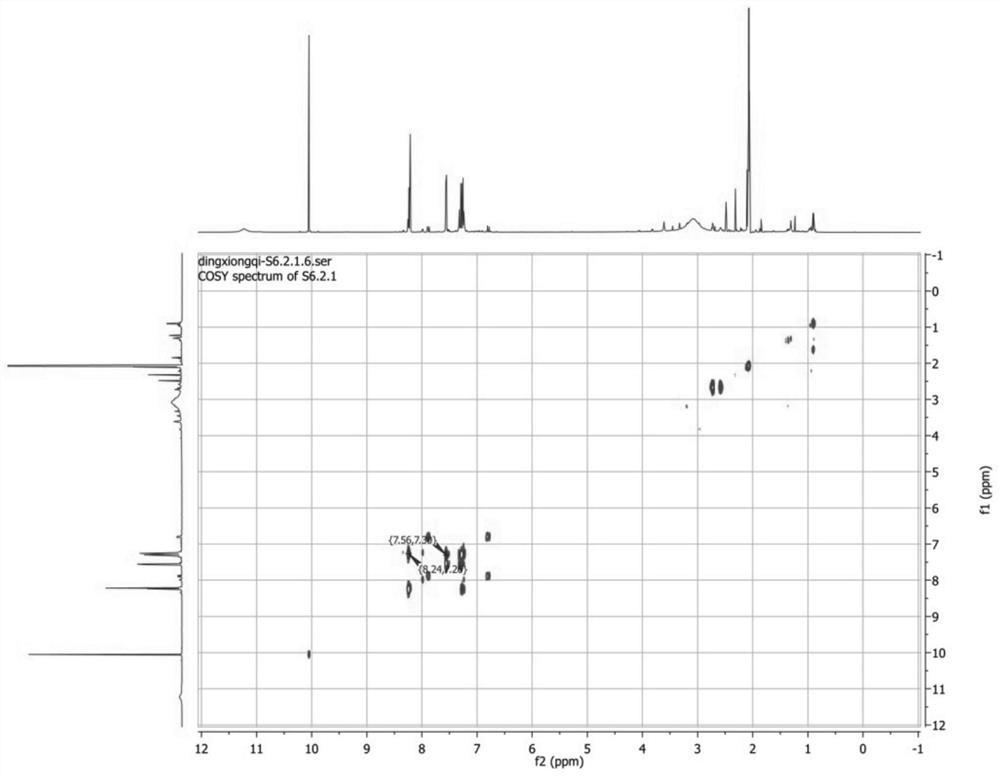
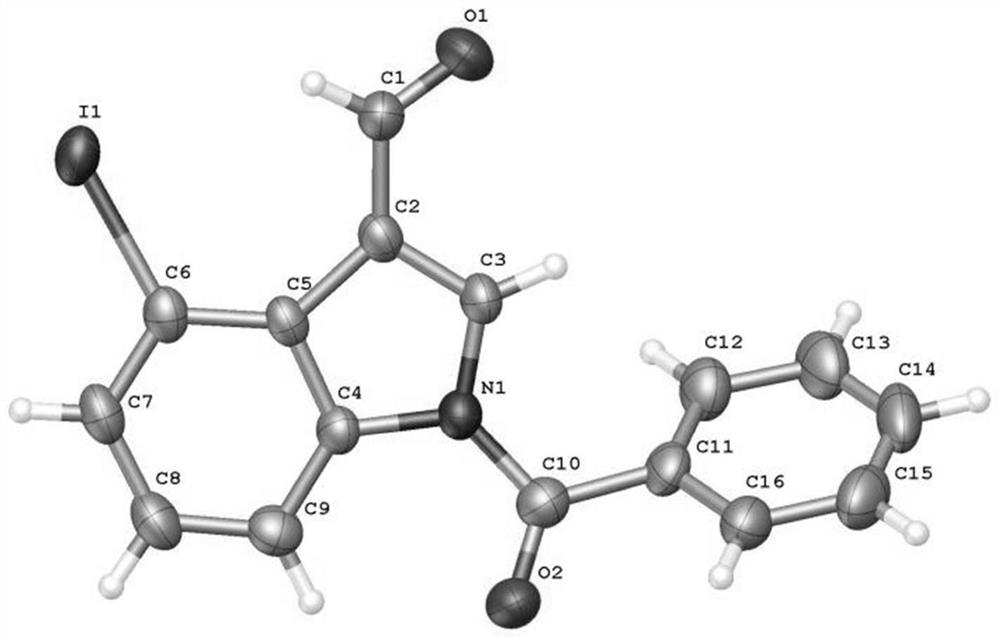
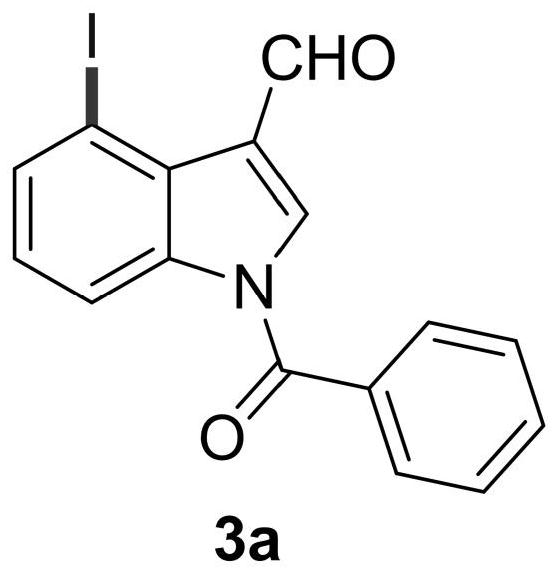
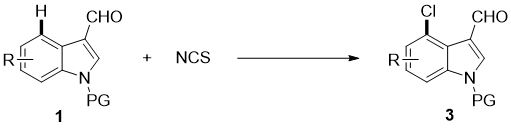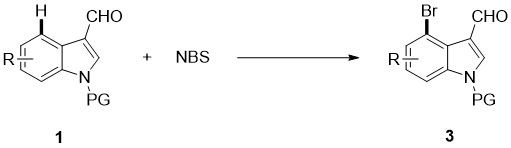Patents
Literature
34 results about "Indolecarboxaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
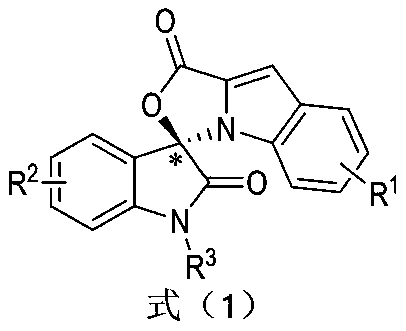
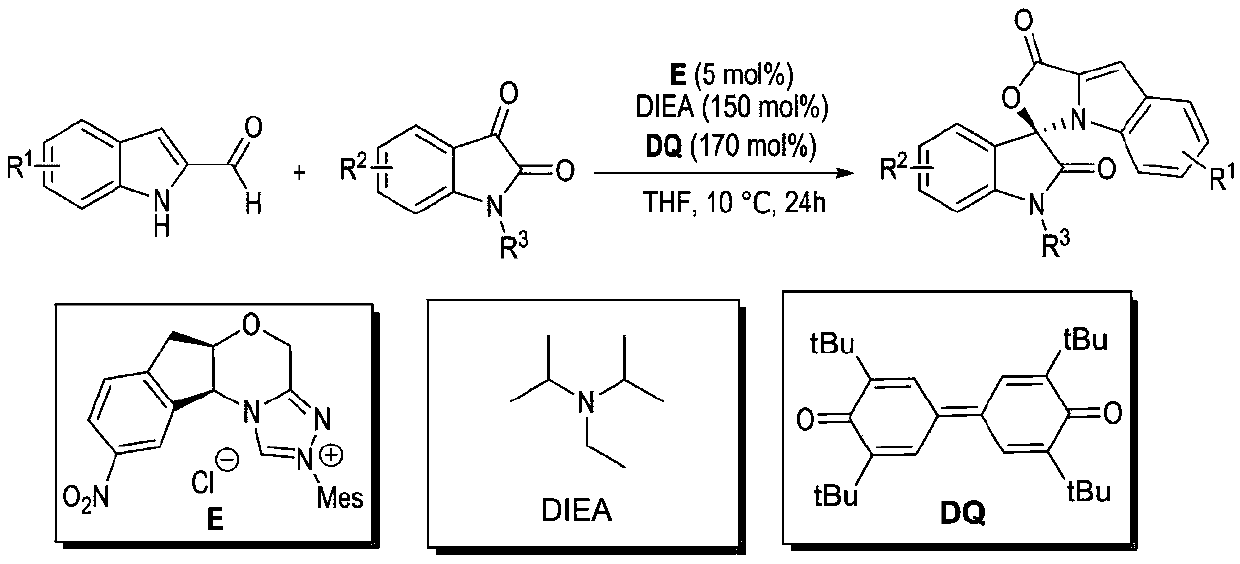
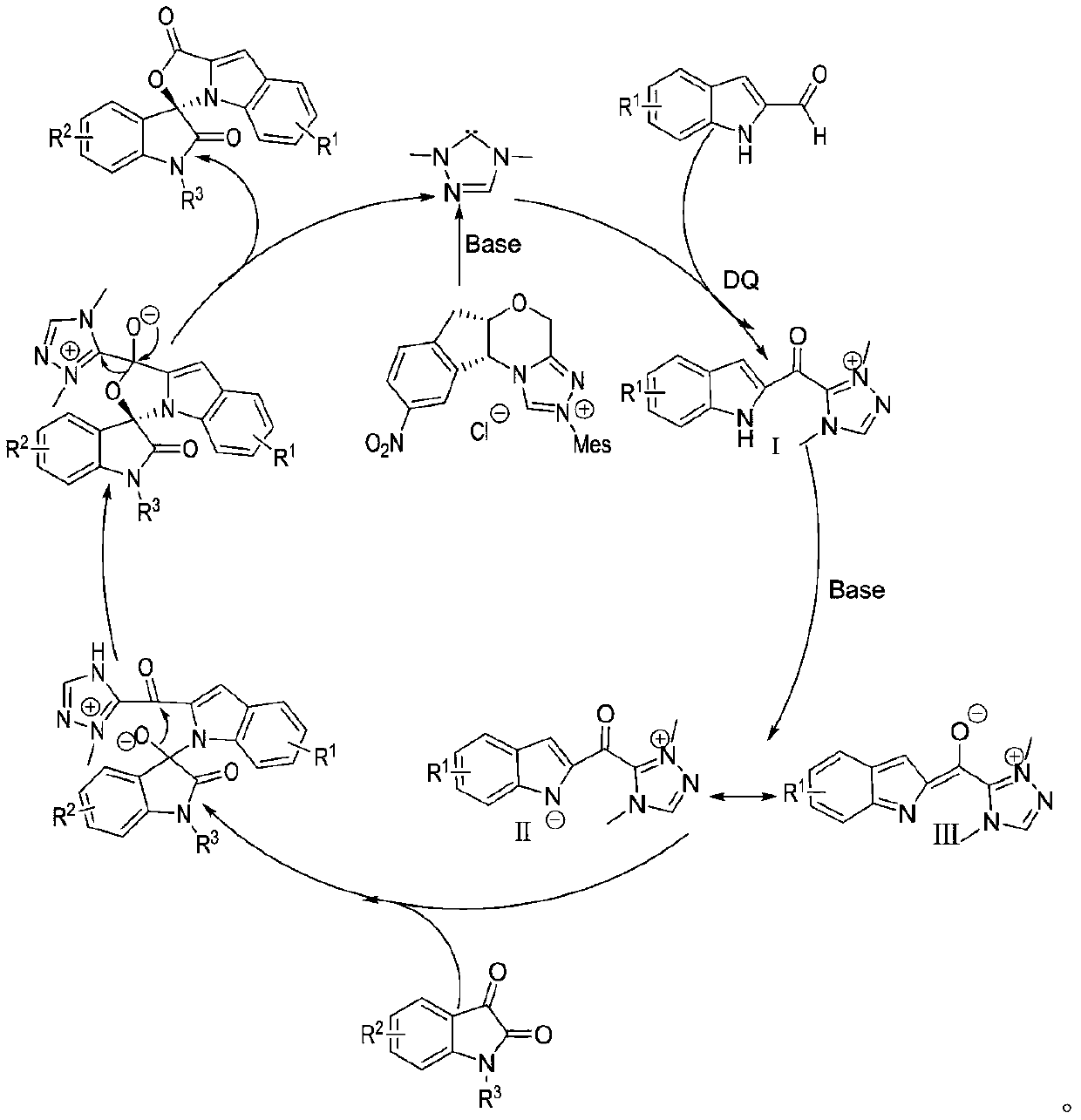
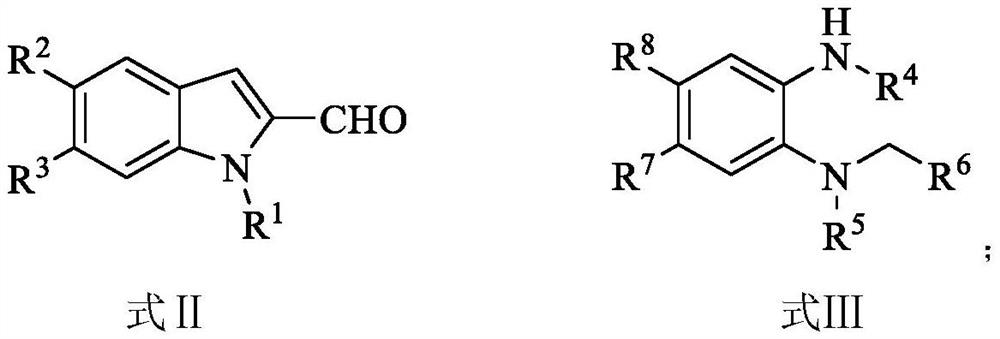
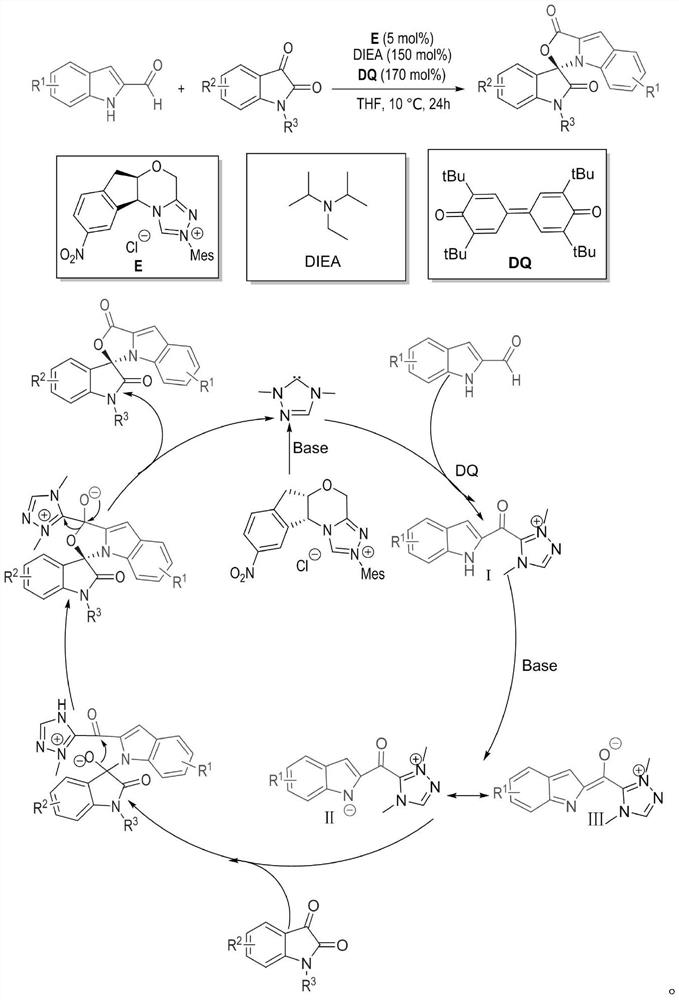
Preparation method and use of n-heterocyclic carbene catalyzed indole-containing skeleton chiral spiro compound
ActiveCN110551136AHigh enantioselectivityImprove universalityBiocideOrganic chemistry methodsKetoneCarbene
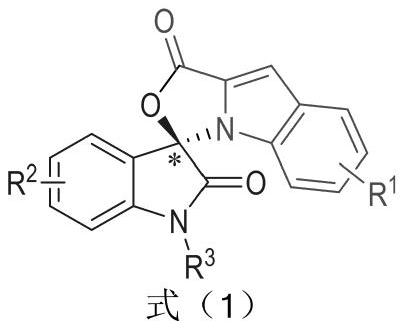
The invention relates to a preparation method for the synthesis of an indole-containing skeleton chiral spiro compound (R)-1-aryl-1'H-spiro[dihydroindole-3,3'-oxazolo[3,4-alpha] indole]-1',2 diketonederivative by n-heterocyclic carbene with extremely small molecule catalysis and high enantioselectivity and a good biological activity use. A general structure formula is shown as follows (please seespecification for the formula), wherein R1 is a substituent of indole-2-formaldehyde, R2 is a substituent of indole-2,3-diketone, and R3 is a different protecting group of the indole-2,3- diketone, methyl, benzyl, and triphenylmethyl. The indole skeleton chiral spiro compound (R)-1-aryl-1'H-spiro[dihydroindole-3,3'-oxazolo[3,4-alpha] indole]-1',2 diketone derivative prepared by an asymmetric cyclization reaction has good universality, excellent yield which reaches up to 98%, enantioselectivity which reaches up to 99 % and good biological activity.
Owner:GUIZHOU UNIV
Indole-3-carboxaldehyde isobutyryl hydrazone derivatives and preparation method thereof
InactiveCN102627597AGood tumor performanceOrganic chemistryAntineoplastic agentsHydrazonePharmaceutical medicine
The invention relates to a new class of indole derivatives and a preparation method thereof. The method comprises the steps of: taking indole-3-carboxaldehyde derivatives and isobutyryl hydrazine as raw materials, and partially or wholly introducing groups with anticancer activities such as -OCH3, -C=N-NH-C=O, and the like to proper positions of the indole derives through chemical reaction to obtain a series of indole compounds containing multiple functional groups. The technology is with simple operation, requires mild reaction conditions and has a high yield. Furthermore, the compounds havegood biological activities, can be used for tumour therapy and have a wide application prospect in a pharmaceutical field.
Owner:QILU UNIV OF TECH
Purine parent-based fluorescent probe compound as well as preparation method and application thereof
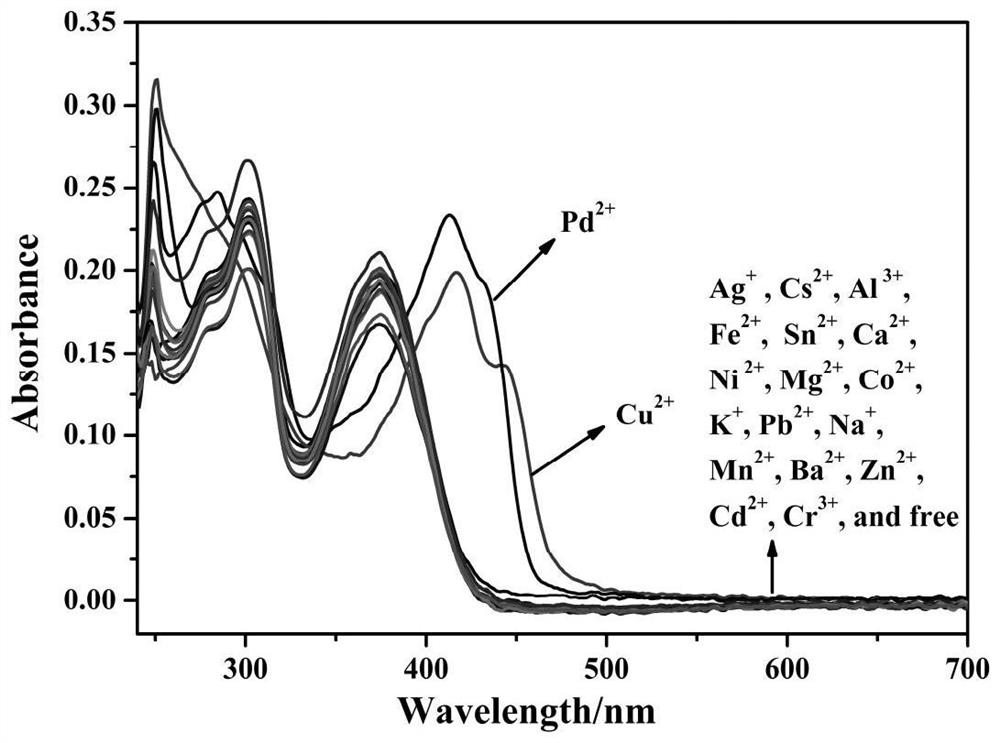
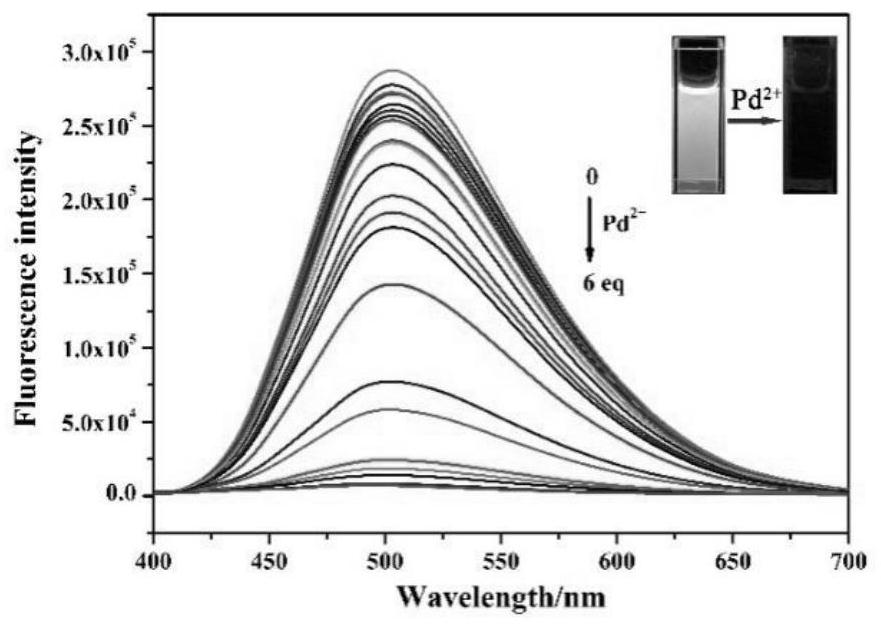
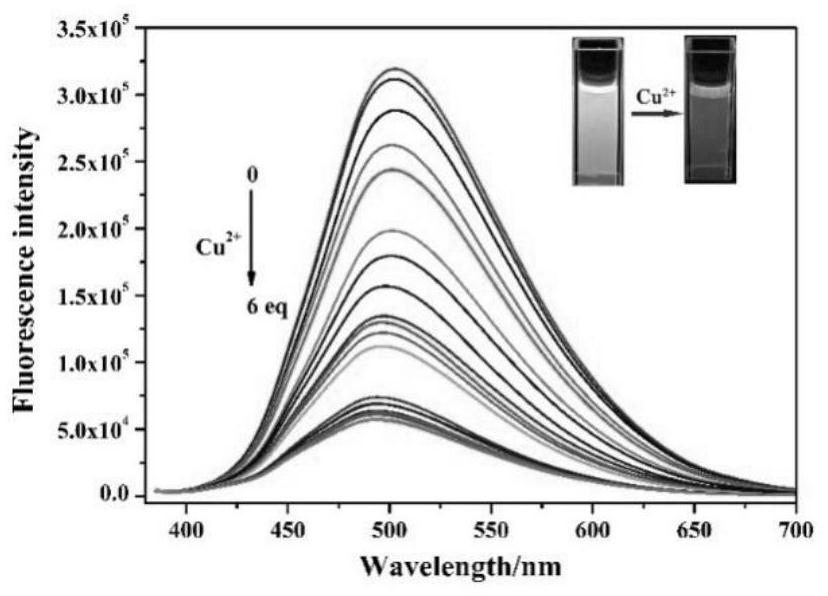
ActiveCN112341463AHigh selectivityGood choiceOrganic chemistryFluorescence/phosphorescenceFluoProbesFluorescence
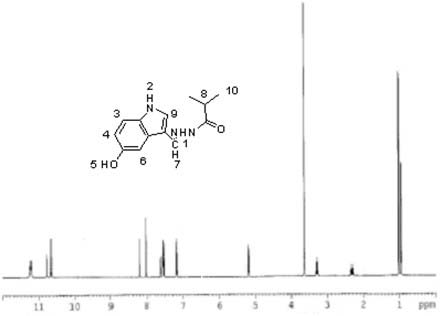
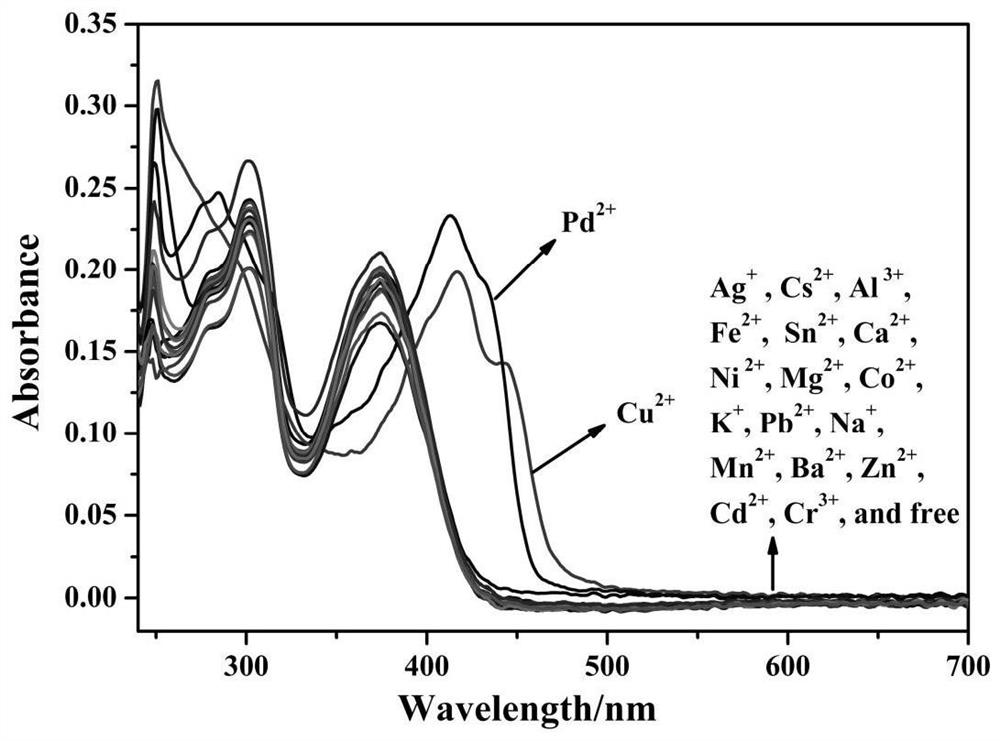
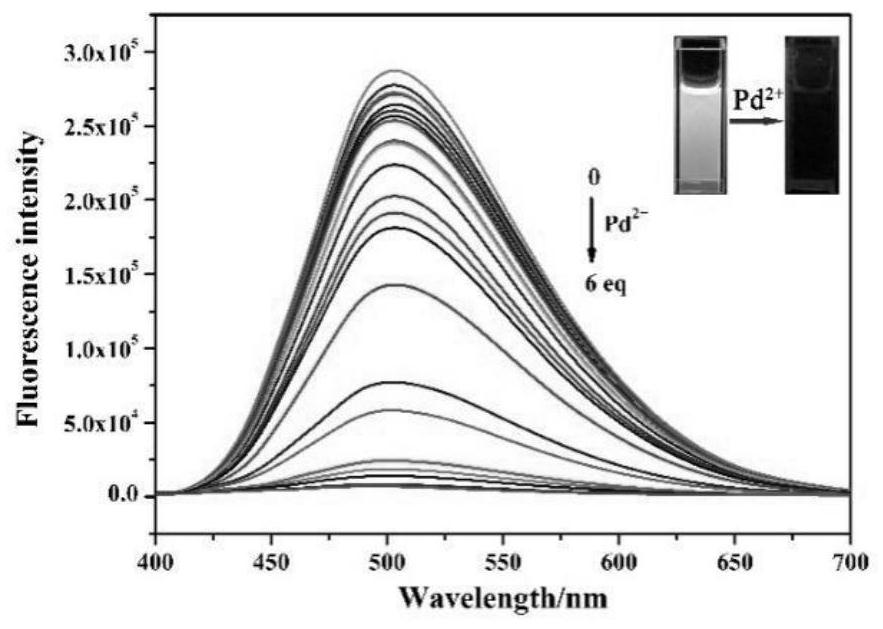
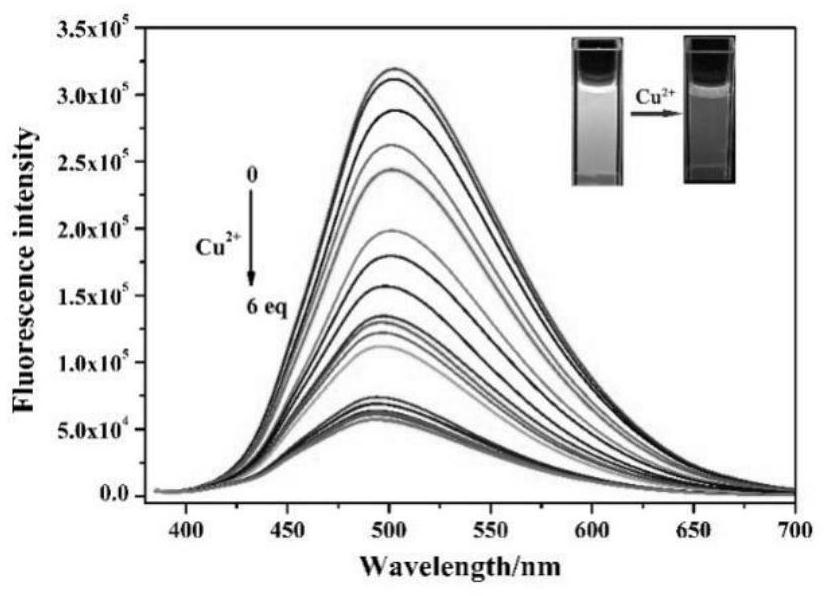
The invention discloses a purine parent-based fluorescent probe compound, and further discloses a preparation method of the fluorescent probe compound and application of the fluorescent probe compoundto detection of palladium ions and copper ions. According to the invention, a purine ring and 3-indolecarboxaldehyde are used as fluorescent groups, hydrazine hydrate is used as a linking group, thepurine parent-based fluorescent probe compound is synthesized, and the obtained fluorescent probe compound has high selectivity and high detection sensitivity on copper and palladium ions, so that thefluorescent probe compound has the advantages of stable structure, good selectivity, high sensitivity and low toxicity; the preparation method is simple in step, raw materials are easy to obtain, andthe obtained product is solid powder and easy to store.
Owner:JIANGSU UNIV OF SCI & TECH
Method for producing indole derivative
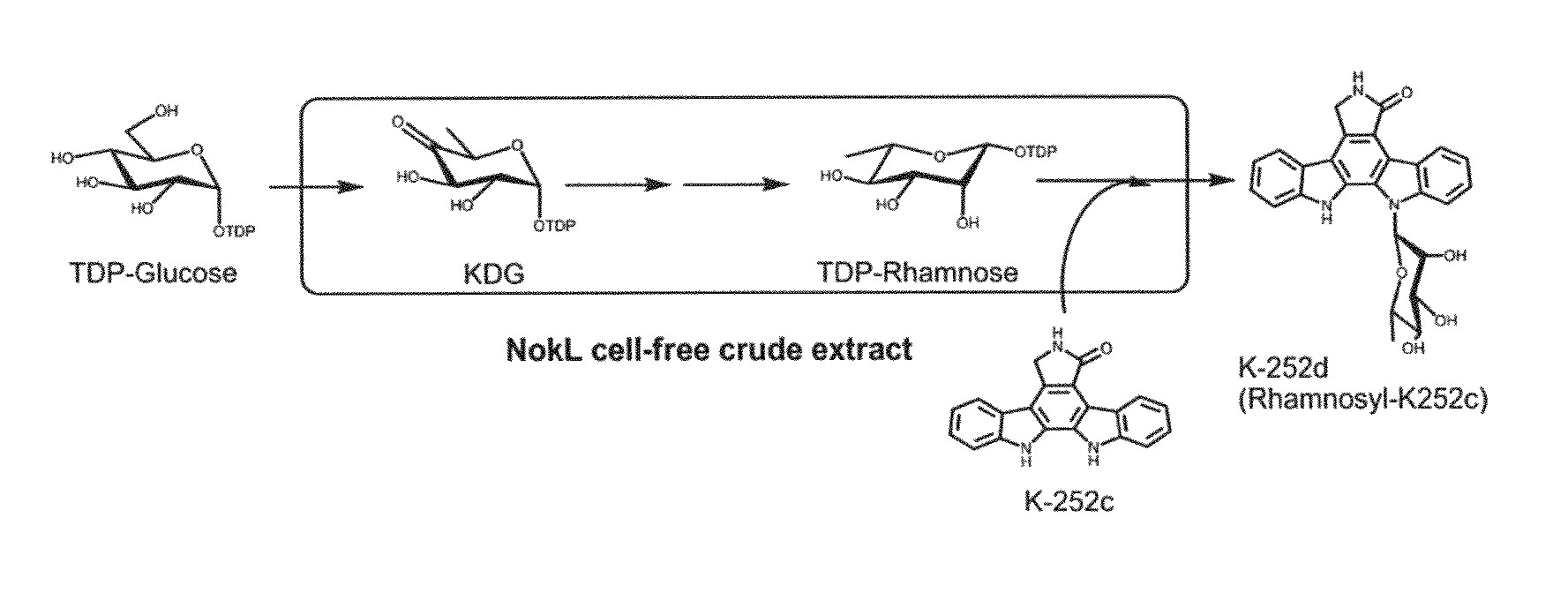
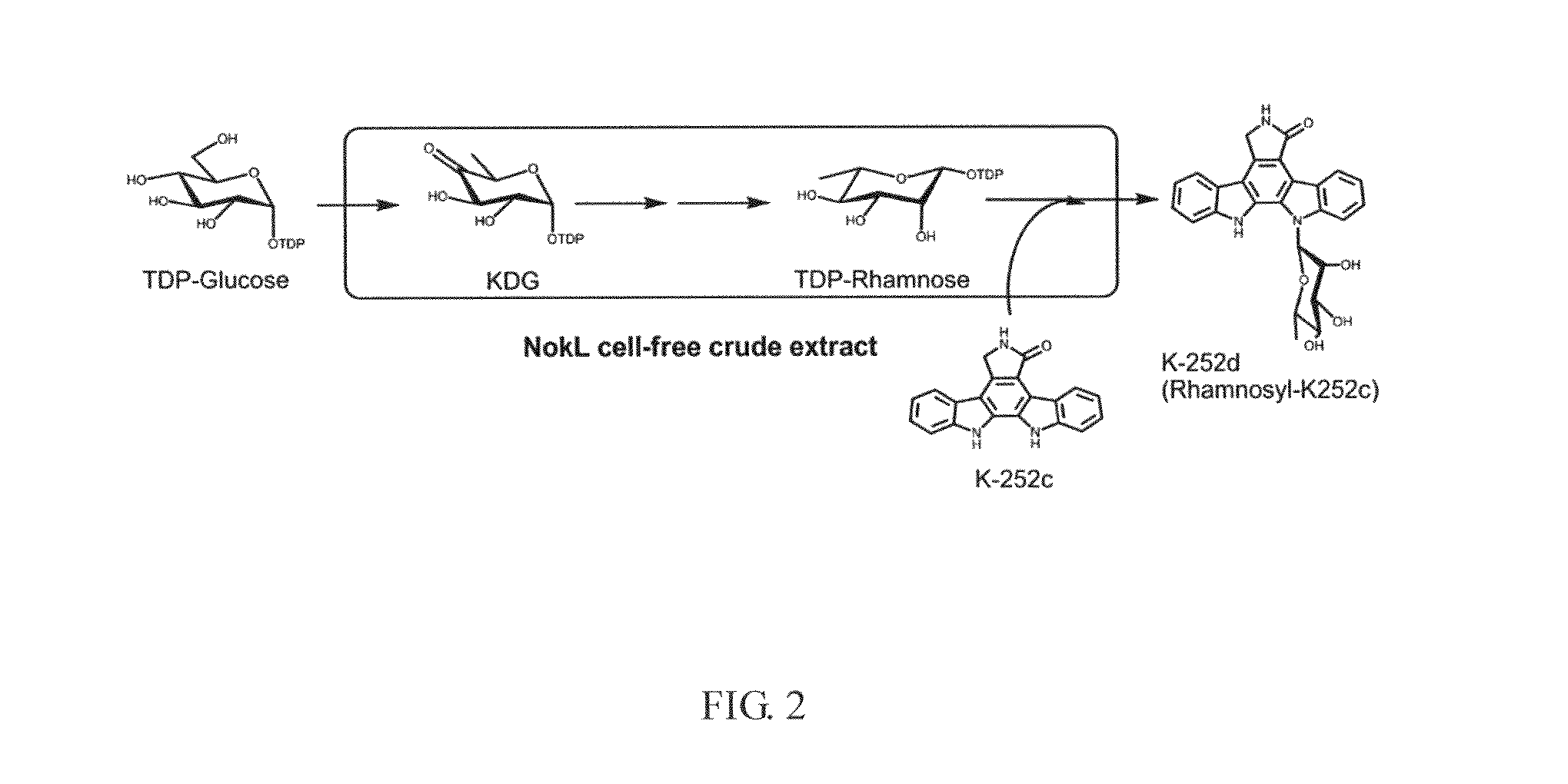
The present invention provides a method for in vitro producing an indole derivative in a one-pot reaction. The method for producing a rhamnosylated indolocarbazole compound includes the steps of transforming a plasmid carrying a gene encoding N-glycosyltransferase into a bacterial strain; expressing the gene encoding N-glycosyltransferase in the bacterial strain; lysing the bacterial strain to obtain a crude enzyme extract; and adding TDP-glucose, an indolocarbazole aglycone and a metal ion in the crude enzyme extract for performing an enzymatic reaction to form the rhamnosylated indolocarbazole compound. Alternatively, the method for producing an indole-3-carboxaldehyde analog includes the steps of transforming a plasmid carrying a gene encoding NokA of Nocardiopsis sp. K-252 into a bacterial strain; expressing the gene encoding NokA in the bacterial strain; lysing the bacterial strain to obtain a crude enzyme extract; and adding an L-tryptophan analog for performing an enzymatic reaction to form the indole-3-carboxaldehyde analog.
Owner:NATIONAL CHIAO TUNG UNIVERSITY
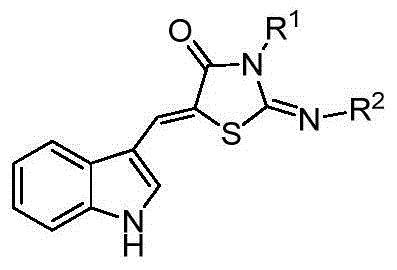
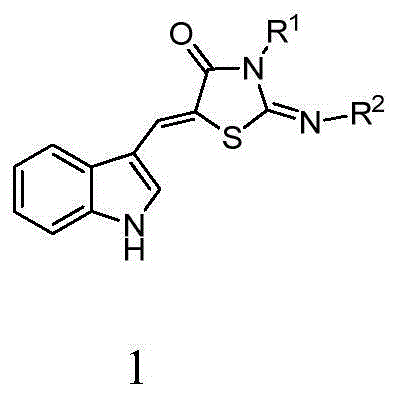
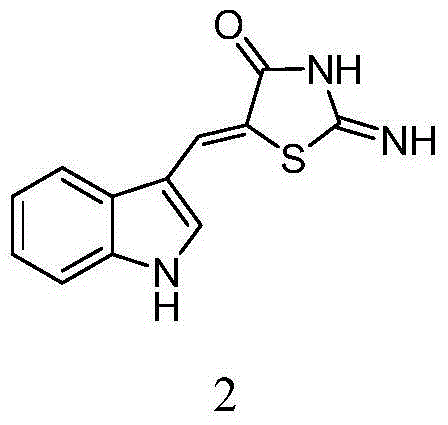
5-(1H-indolyl-3-methylene)-1,3-thiazolidinyl-4-one derivatives, and synthesis method and application thereof
InactiveCN104059060ABroad biological activityHigh activityOrganic active ingredientsOrganic chemistryThioureaChloroacetic acids
The invention relates to 5-(1H-indolyl-3-methylene)-1,3-thiazolidinyl-4-one derivatives, and a synthesis method and application thereof. By using ethanol and / or water as a solvent, substituted 2-substituted-imino-1,3-thiazolidinyl-4-one and 1H-indolyl-3-formaldehyde are subjected to reflux reaction under the catalytic condition of piperidine through intermolecular dehydration condensation reaction to form methylene linking group, thereby obtaining the 5-(1H-indolyl-3-methylene)-1,3-thiazolidinyl-4-one derivatives. The intermediate 2-substituted-iminothiazolidinyl-4-one is prepared by carrying out cyclization reaction on various monosubstituted ethyl thiocarbamide chloroacetates or chloroacetic acids in a low-boiling solvent under reflux conditions, and the intermediate 2-substituted-imino-3-substituted-1,3-thiazolidinyl-4-one is prepared by carrying out a green environment-friendly synthesis technique on various disubstituted symmetric thiocarbamides and chloroacetic acids. The bioactivity preliminary screening experiment result of all the target compounds on the enzyme molecular level indicates that the target products have certain inhibition activity on PTP1B and CDC25B to different degrees.
Owner:XI AN JIAOTONG UNIV +1
Indole eight-membered middle-ring compound and preparation method thereof
InactiveCN113121548AImprovements to the problem of acceptors being restricted to electron-deficient double bondsMild conditionsBiocideOrganic chemistryOrganic synthesisCombinatorial chemistry
The invention discloses an indole eight-membered middle-ring compound and a preparation method thereof, which belong to the technical field of organic synthesis. Indole-2-formaldehyde and N-substituted aniline are subjected to an in-situ reaction to initiate [1, 6]-HT, and generated high-activity imine positive ions and electronegative indole C-3 are subjected to a Friedel-Crafts reaction to synthesize the indole diaza eight-membered fused ring compound. The reaction has the advantages of simple and easily available raw materials, strong universality, mild reaction conditions, good chemical selectivity, high product yield and the like.
Owner:QINGDAO AGRI UNIV
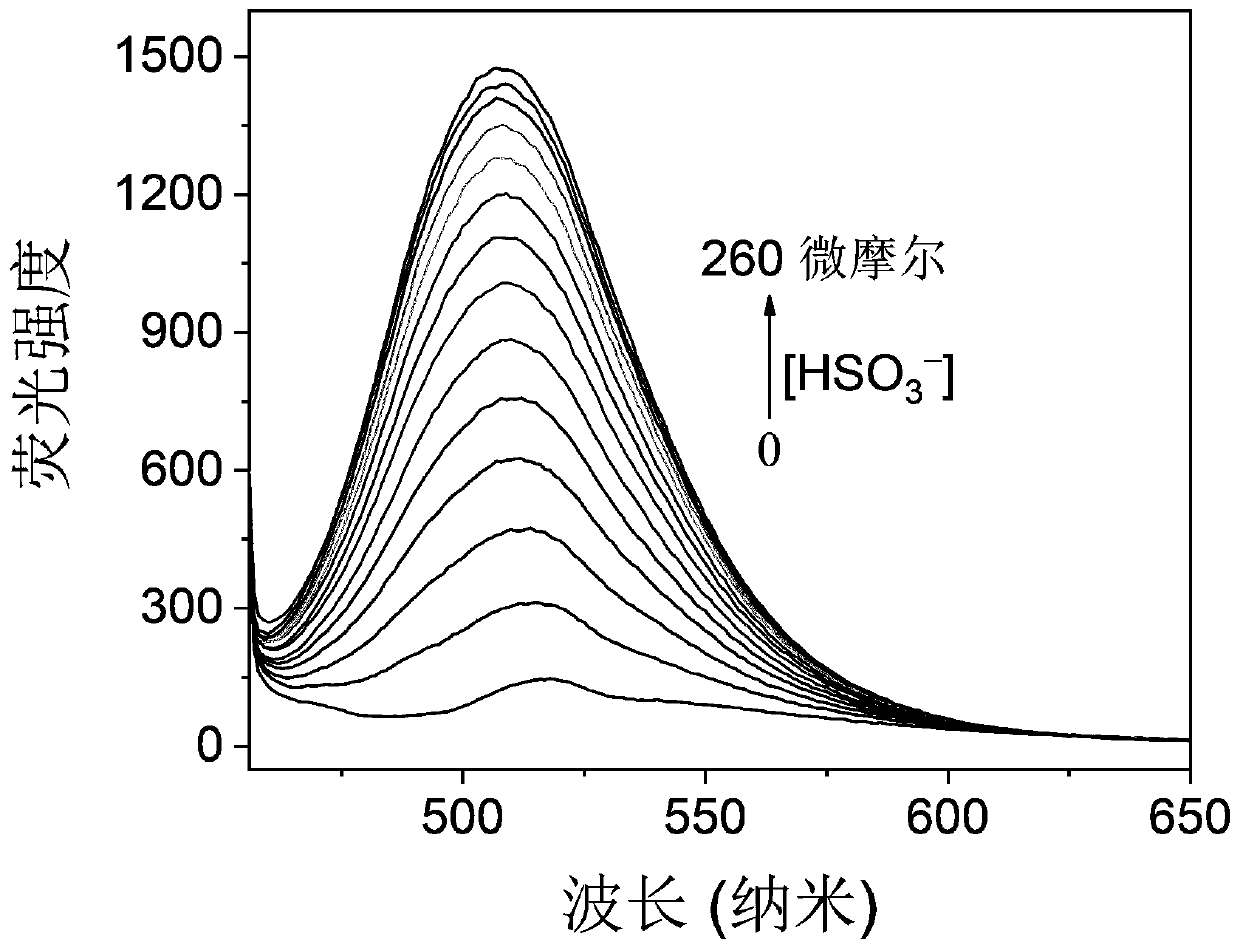
Turn-on type fluorescent probe for rapidly detecting sulfite(bisulfite)as well as synthesis method and application of turn-on type fluorescent probe
ActiveCN110563708AShort response timeHigh measurement sensitivityOrganic chemistryFluorescence/phosphorescenceFluoProbesPhotochemistry
The invention relates to a turn-on type fluorescent probe for rapidly detecting sulfite(bisulfite). The molecular formula of the fluorescent probe is C21 + nH15 + mN1 + xO3 + y, wherein n, m, x and yare all integers from 1 to 20, and the molecule has the following structure: substituent R1 is one of H, alkyl, aryl, nitryl, ester group, ether group and diethylamino, and n in the 3-indolecarboxaldehyde derivative is an integer from 1 to 20. The fluorescent probe is short in response time(10 seconds), high in measurement sensitivity, simple in molecular structure and simple and convenient in synthesis method, and can be used for cell imaging and detection of sulfite(bisulfite)in food and traditional Chinese medicinal materials, so that the fluorescent probe is extremely easy to popularize and apply practically.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Chilled fresh meat freshness marker and screening and prediction model fitting method and application thereof
ActiveCN111830181AAccurate detectionHigh resolutionComponent separationCharacter and pattern recognitionBiotechnologyMetabolite
The invention discloses a metabonomics-based chilled fresh meat freshness marker and a screening and prediction model fitting method and application thereof. The marker is prepared from indole-3-formaldehyde, uridine monophosphate, phenylmercaptouric acid, gluconic acid, tyramine and serine-phenylalanine. The prediction model is: Y=3.964+1.97E<-7>X1- 4.22E<-7>X2-3.37E<-7>X3+8.80E<-8>X4+1.26E<-8>X5-5.57E<-7>X6. According to the method, an Agilent 1290 UHPLC is connected with a Q Exactive Orbitrap high-resolution mass spectrum in series, the method has higher resolution, more substances can be detected more accurately, metabolites in the preservation process of the chilled fresh chicken can be illustrated more comprehensively, and the obtained result is more reliable.
Owner:YANGZHOU UNIV
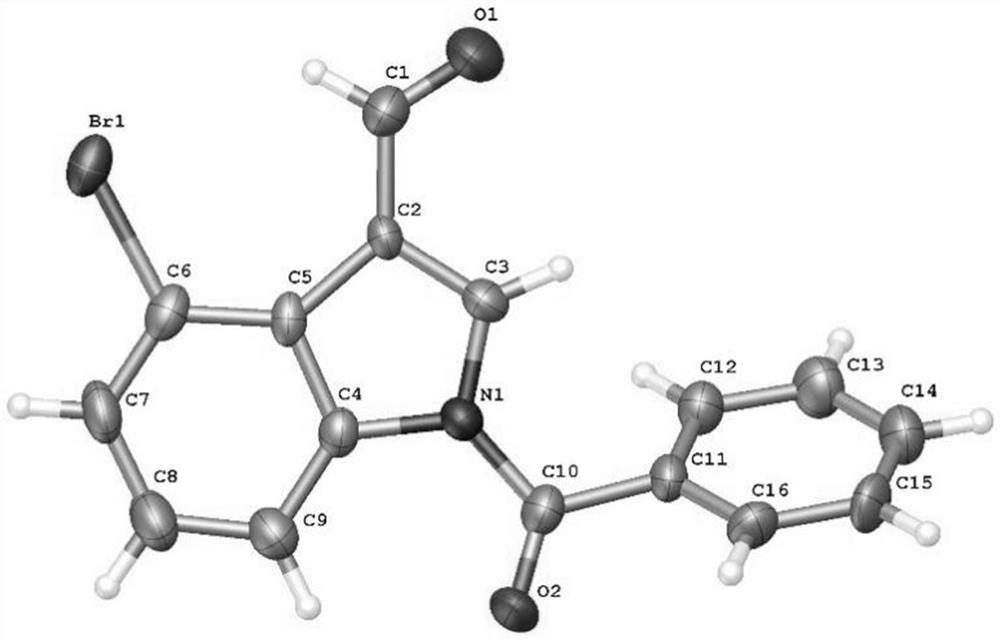
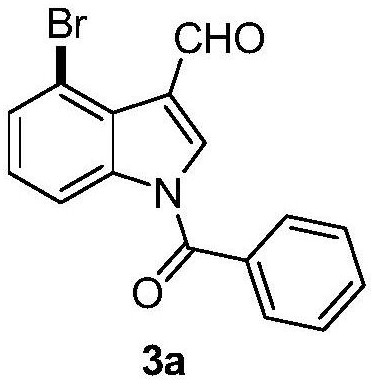
4-bromoindole compound and preparation method thereof
ActiveCN112028812AHigh reaction yieldHigh chemoselectivityAntibacterial agentsNervous disorderImideBiochemical engineering
The invention discloses a 4-bromoindole compound and a preparation method thereof. Various N-protected indolo-3-formaldehyde and N-bromosuccinimide (NBS) are used as reaction substrates to prepare the4-bromoindole compound. The reaction yield can reach medium to excellent, the chemical selectivity and regioselectivity of the reaction are excellent, the reaction conditions are mild, and the application range of a substrate is wide. The method has the advantages of simple operation, low cost, few side reactions, high product purity, convenient separation and purification, and suitableness for large-scale preparation, so the obtained product has a very good application prospect in the field of biological medicines.
Owner:PINGDINGSHAN UNIVERSITY +1
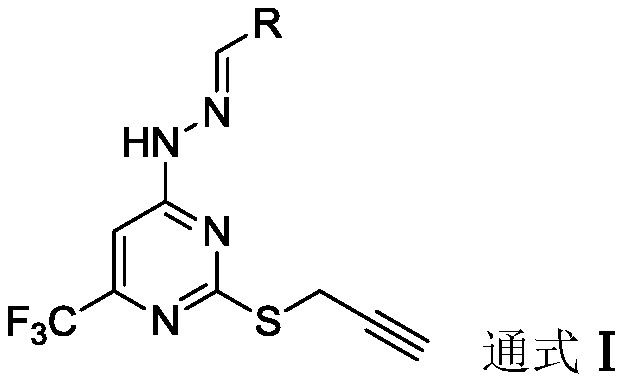
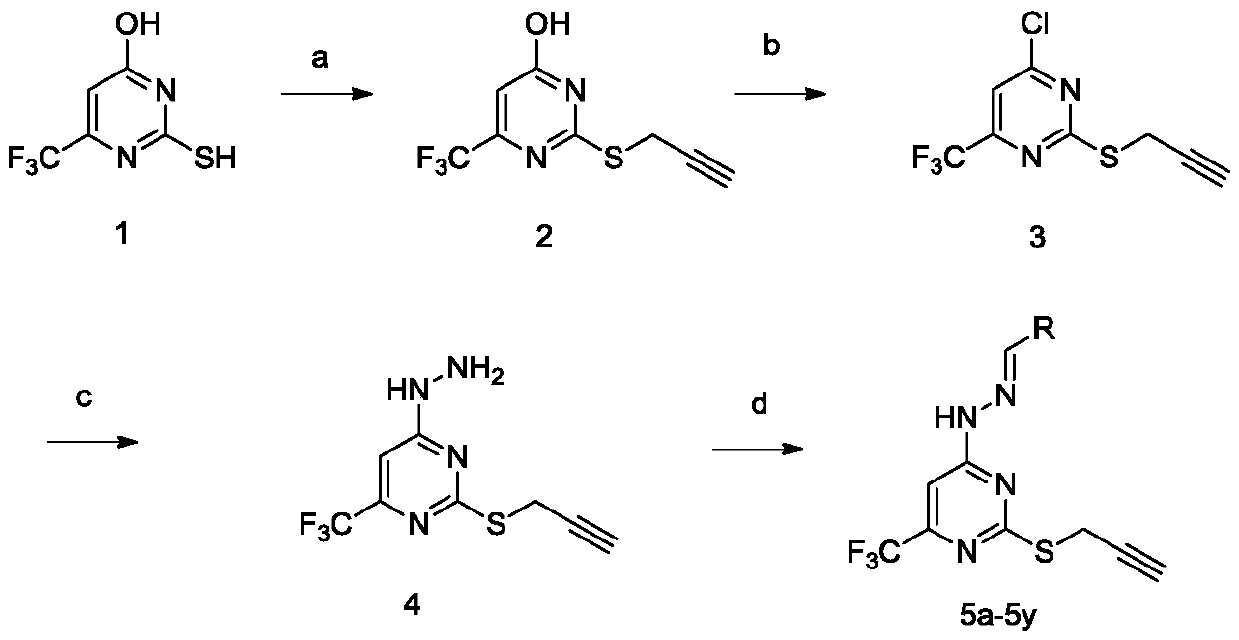
Trifluoromethyl pyrimidine derivatives containing Schiff base structural unit, and preparation method and application thereof
ActiveCN111187220AEnhanced inhibitory effectHigh activityOrganic chemistryAntineoplastic agentsBenzaldehydeMethyl group
The invention belongs to the field of medicinal chemistry, and discloses Schiff base structural unit-containing trifluoromethyl pyrimidine derivatives with antitumor activity, and a preparation methodand application thereof. The compounds have structures as shown in a general formula I which is described in the specification. In the general formula I, R is a benzaldehyde group, a substituted benzaldehyde group, a 9-anthraaldehyde group or a 3-indole formaldehyde group. Results of in-vitro anti-tumor activity evaluation show that the series of derivatives have obvious inhibiting and killing effects on various tumor cells. The derivatives can be further optimized and developed into novel drugs to be applied to clinical prevention and treatment of cancers.
Owner:ZHENGZHOU UNIV
Indole-3-carboxaldehyde isobutyryl hydrazone derivatives and preparation method thereof
InactiveCN102627597BGood tumor performanceOrganic chemistryAntineoplastic agentsHydrazoneChemical compound
Owner:QILU UNIV OF TECH
Method for separating and purifying methyl p-hydroxybenzoate and 3-indolylformaldehyde from Trichosanthes kirilowii Maxim stem and leaf
InactiveCN103467297ASimple compositionExtended service lifeOrganic compound preparationCarboxylic acid esters separation/purificationBenzoic acidMethanol water
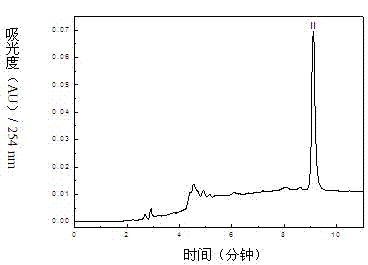
The invention relates to a method for separating and purifying methyl p-hydroxybenzoate and 3-indolylformaldehyde from Trichosanthes kirilowii Maxim stem and leaf, which uses Trichosanthes kirilowii Maxim stem and leaf as the raw material and comprises the following step: (1) preparation of Trichosanthes kirilowii Maxim stem and leaf crude extract; (2) extraction; (3) reversed phase C18 column separation and purification; and (4) semipreparative high performance liquid chromatography separation and purification: carrying out separation and purification by semipreparative high performance liquid chromatography by using methanol-water as a mobile phase to obtain the two high-purity components, which are respectively methyl p-hydroxybenzoate and 3-indolylformaldehyde after identification. The technical process is green and environment-friendly, does not have severe damage to the environment, and is low in comprehensive cost.
Owner:LIAOCHENG UNIV
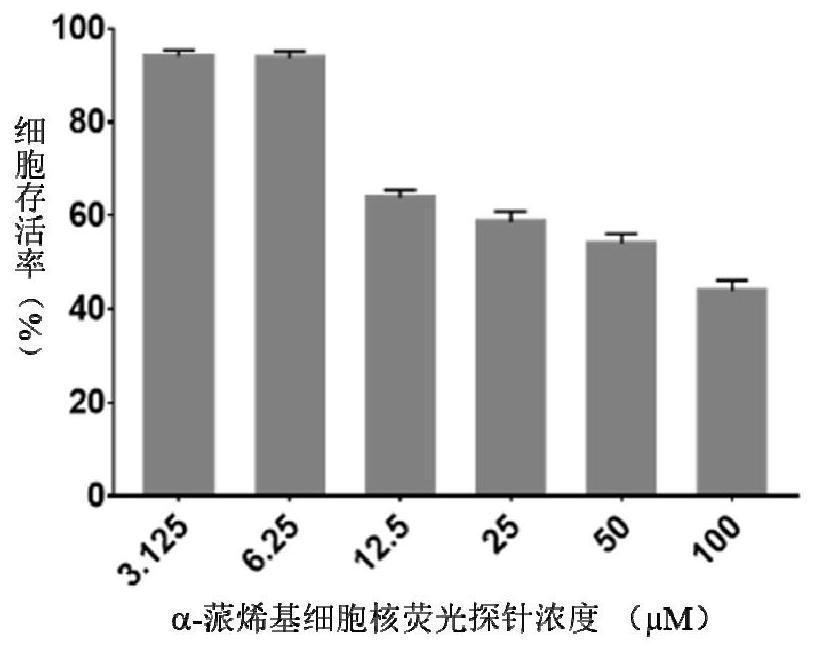
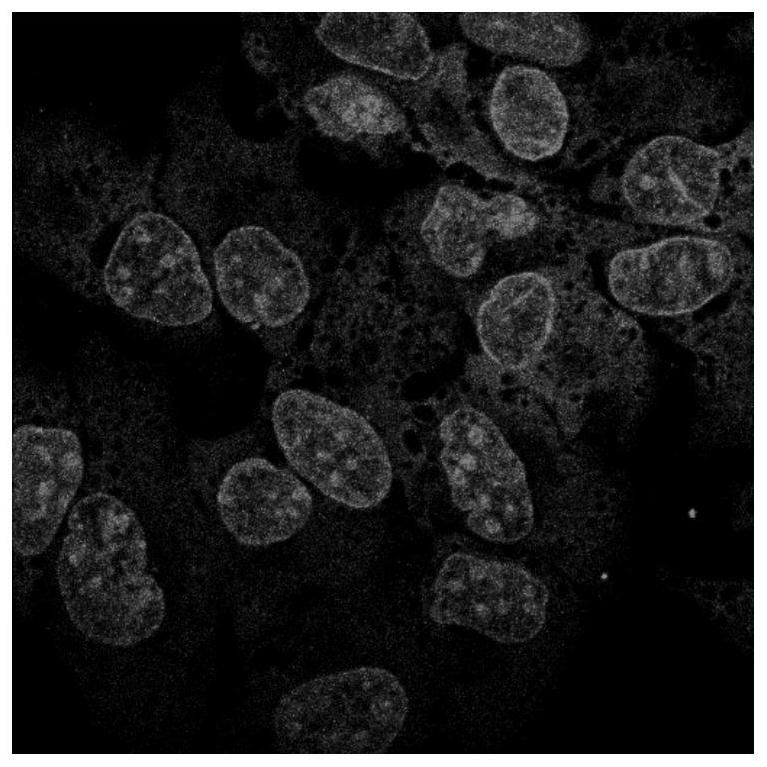
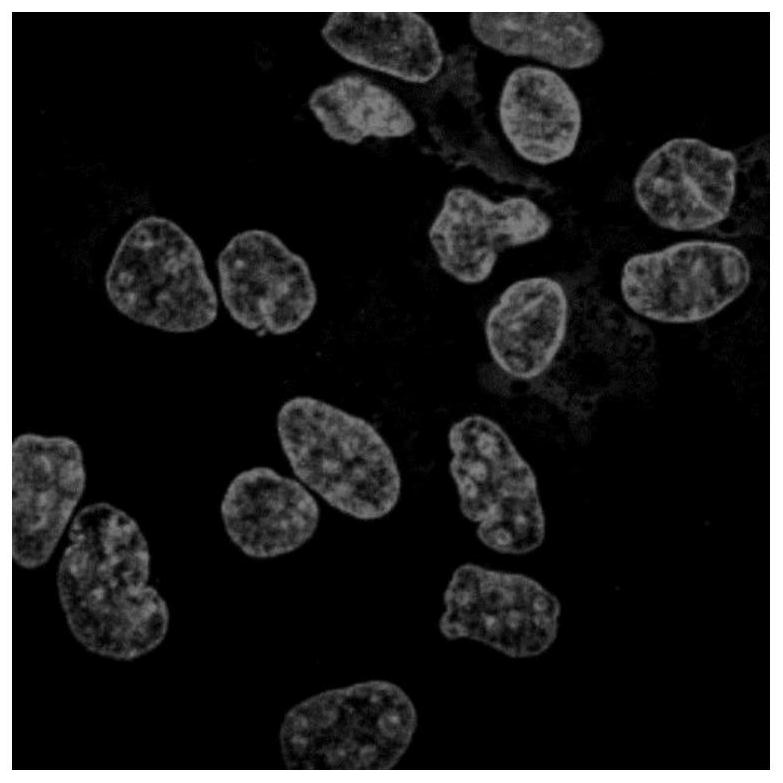
A kind of α-pinene-based nuclear fluorescent probe and its preparation method and application
Owner:NANJING FORESTRY UNIV
Application of Indole-3-Carboxaldehyde and Its Derivatives in Controlling Plant Diseases Caused by Phytopathogenic Fungi
The invention discloses the application of indole-3-formaldehyde and its derivatives in preventing and treating plant diseases caused by plant pathogenic fungi. The invention provides the application of indole-3-carboxaldehyde or its derivatives or pharmaceutically acceptable salts in the prevention and treatment of plant diseases caused by phytopathogenic fungi, or in the preparation of pesticides for the prevention and control of plant diseases caused by phytopathogenic fungi Or application in biocontrol agents. The present invention proves that indole-3-formaldehyde and derivatives thereof have good inhibitory activity to phytopathogenic fungi through the measurement of bacteriostatic activity, and it has a good inhibitory effect on the growth and sexual coordination of phytopathogenic fungi, which can inhibit The formation of dikaryotic mycelia prevents phytopathogenic fungi from normally infecting plants, thereby effectively inhibiting the occurrence of plant fungal diseases, providing a reference for green prevention and control of plant diseases caused by phytopathogenic fungi, and has broad application prospects.
Owner:SOUTH CHINA AGRI UNIV
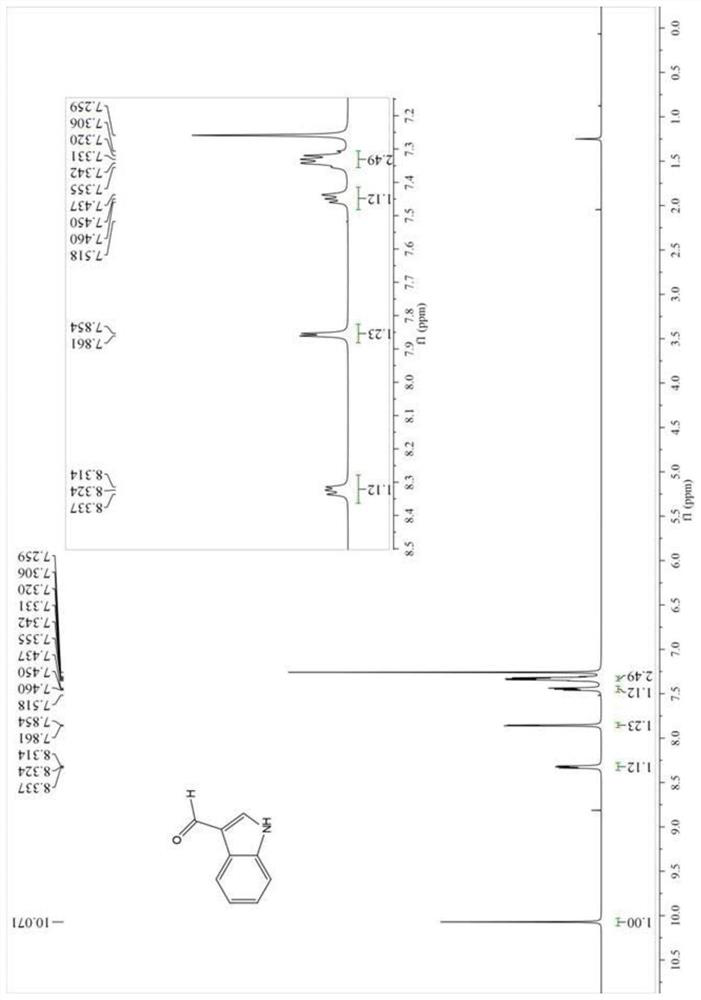
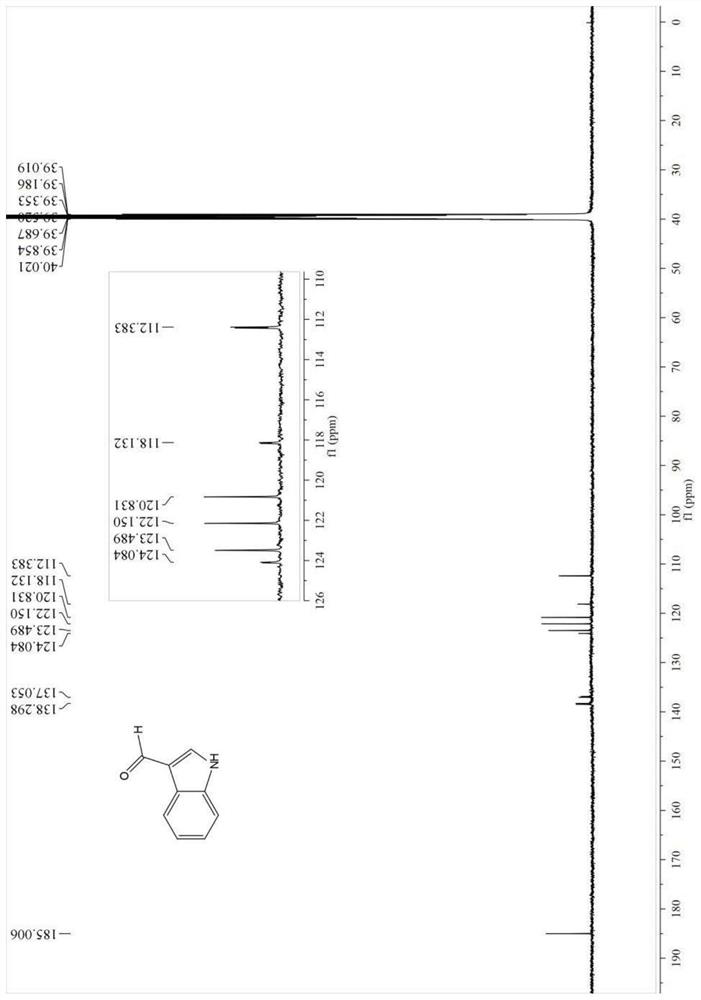
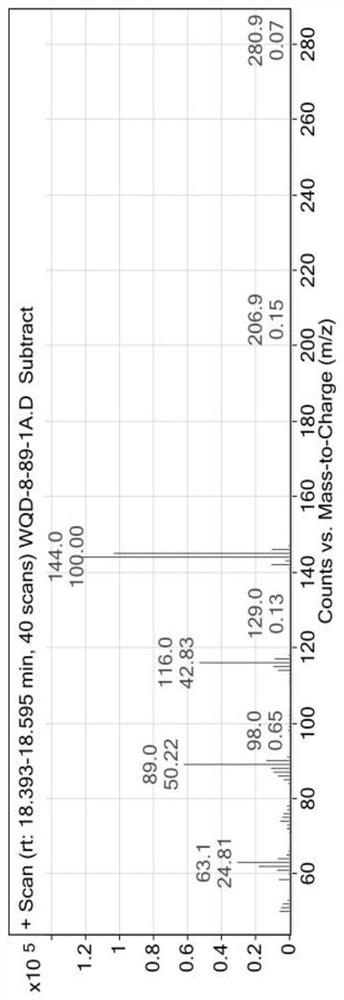
4-position chloroindole compound and its preparation method
ActiveCN112028813BHigh chemoselectivityHigh regional selectivityOrganic chemistryImideRegioselectivity
The invention discloses a 4-position chloroindole compound and a preparation method thereof. Various N-protected indole-3-formaldehydes and N-chlorosuccinimide (NCS) were used as reaction substrates to prepare 4-position chloroindole compounds. The reaction yield can reach moderate to excellent, the reaction chemoselectivity and regioselectivity are excellent, the reaction conditions are mild, and the substrate has a wide range of application; it is easy to operate, low in cost, less in side reactions, high in product purity, and easy to separate Purification and can be applied to large-scale preparation, so the resulting product has a very good application prospect in the field of biomedicine.
Owner:PINGDINGSHAN UNIVERSITY +1
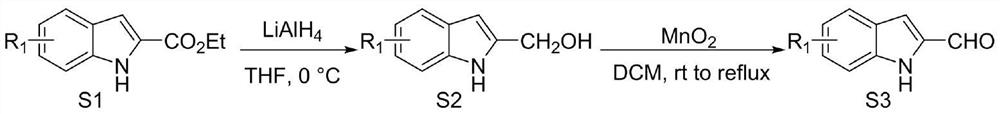
A method for synthesizing indole-3-carboxaldehyde compounds
ActiveCN108329249BEfficiently obtainedBroad compatibilityOrganic chemistryPtru catalystHexamethylenetetramine
The invention relates to a method for synthesizing indole-3-carboxaldehyde compounds, and belongs to the technical field of organic synthesis. The method comprises the following steps: mixing an indole compound, hexamethylenetetramine, crystal aluminum trichloride and N,N-dimethylformamide in proportion, performing a reaction at the temperature of 120 DEG C for 1-20 h, performing reduced-pressurefiltration, performing washing, performing filtration, performing concentration, and performing column chromatography purification to obtain a refined indole-3-carboxaldehyde compound. The method provided by the invention overcomes the shortcomings that preparation of an indole-3-carboxaldehyde compound in the prior art needs to use an unstable peroxide and a reaction is performed for a long timeat high temperature; equipment adopted in the method is simple, a product yield is higher, and a yield of the obtained target product can reach 94%; in addition, the method has low requirements on reaction conditions, a less use amount of a catalyst, low energy consumption and a simple post-treatment process which is easy to operate, and does not need to use a large amount of an acid or an alkali;a post-treatment solvent can be recycled and less industrial three waste (waste water, waste gas and solid waste) is discharged; and the method is suitable for large-scale production.
Owner:盐城锦明药业有限公司 +1
Indole-4-formaldehyde compound with bacteriostatic activity in taxillus chinensis, and preparation method and application of indole-4-formaldehyde compound
ActiveCN112321479AGood antibacterial effectEasy to prepareAntibacterial agentsOrganic active ingredientsBiotechnologyOrganosolv
The invention discloses a compound with antibacterial activity in taxillus chinensis, a preparation method and application of the compound in preparation of antibacterial drugs, and belongs to the field of phytochemistry. The preparation method comprises the following steps of: drying taxillus chinensis as a raw material in the sun, crushing the taxillus chinensis, soaking and extracting with an organic solvent, merging extracting solutions, filtering and concentrating to obtain taxillus chinensis extract extractum; then extracting with n-butyl alcohol and dichloromethane, and carrying out vacuum concentration on the dichloromethane extract to obtain dichloromethane extract extractum; and dissolving the dichloromethane extract extractum, stirring, carrying out silica gel column chromatography, concentrating the eluent, carrying out normal phase silica gel chromatographic column (200-300 meshes) separation and purification, and carrying out gel column chromatography separation and purification to obtain a target, namely pure indole-4-formaldehyde. The indole-4-formaldehyde compound has a relatively strong bacteriostatic effect, and the minimal inhibitory concentration MIC value of the indole-4-formaldehyde compound on methicillin-resistant staphylococcus aureus is 128[mu]g / mL, so that the bacteriostatic effect of the indole-4-formaldehyde compound is far superior to that of clindamycin hydrochloride (MIC is equal to 1024[mu]g / mL).
Owner:JILIN UNIV
Synthetic method for indole-3-carboxaldehyde compounds
ActiveCN102786460AReduce separation and purification processEasy to operateOrganic chemistryMethylanilineCombinatorial chemistry
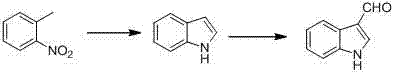
The invention discloses a synthetic method for indole-3-carboxaldehyde compounds. The synthetic method comprises the following steps: (1) using anhydrous dimethylformamide as a solvent, and slowly adding phosphorus oxychloride, and agitating for 30 minutes at 0 to 5 DEG C so as to obtain a Vilsmeier agent; (2) using the anhydrous dimethylformamide as the solvent, adding 2-methylaniline compounds shown in the formula (I), dropping the Vilsmeier agent prepared in step (1) at 0 to 5 DEG C, agitating for 1 to 2 hours at room temperature after dropping, and heating for reflux to react for 5 to 8 hours; and (3) cooling after the reaction, adding a saturated sodium carbonate solution to adjust to alkalinity, filtering the precipitated solid, an drying to obtain a product, and finally recrystallizing the product to obtain the indole-3-carboxaldehyde compounds as shown in the formula (II). The method disclosed by the invention is simple to operate, and low in cost of production, and is not only suitable for small-scale preparation in a laboratory, but also suitable for large-scale industrial production.
Owner:FUJIAN TIANFU BIOTECH DEV CO LTD
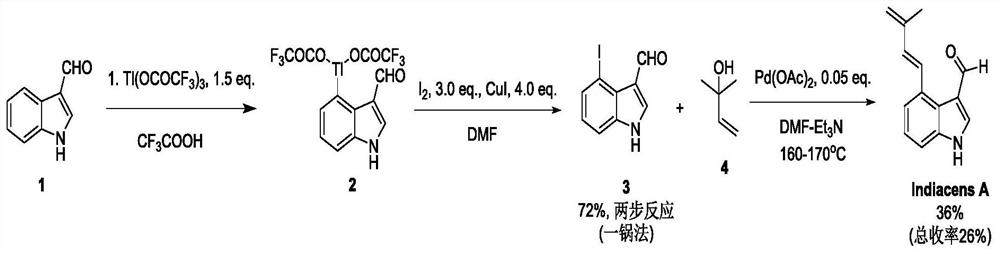
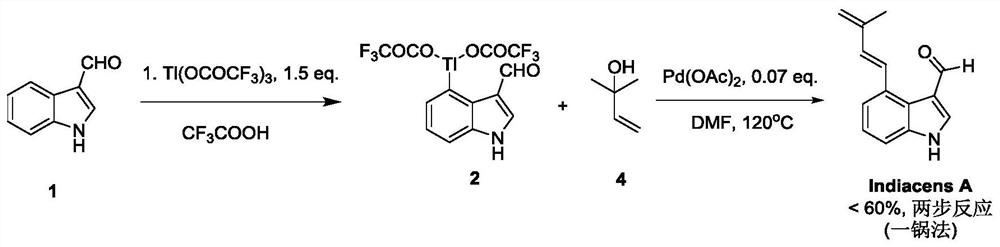
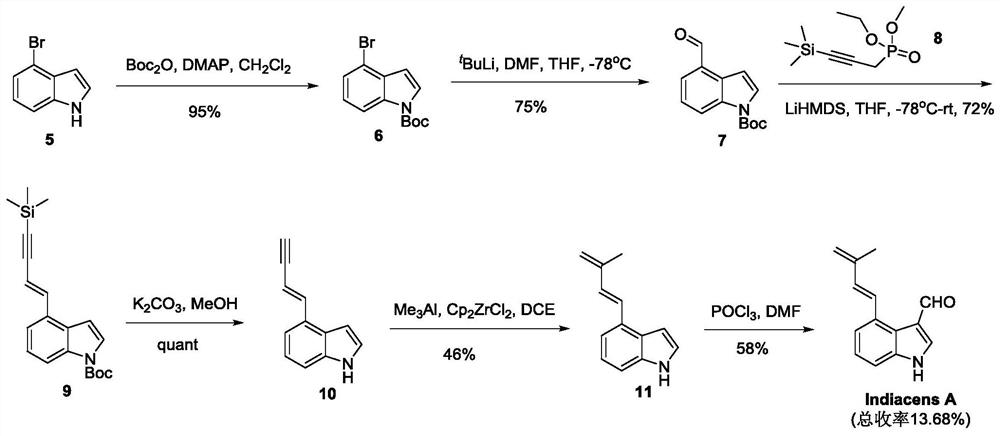
Synthesis of indiacens A
ActiveCN107935905BReduce consumptionReduce usageOrganic chemistryFormaldehyde synthesisOrganic synthesis
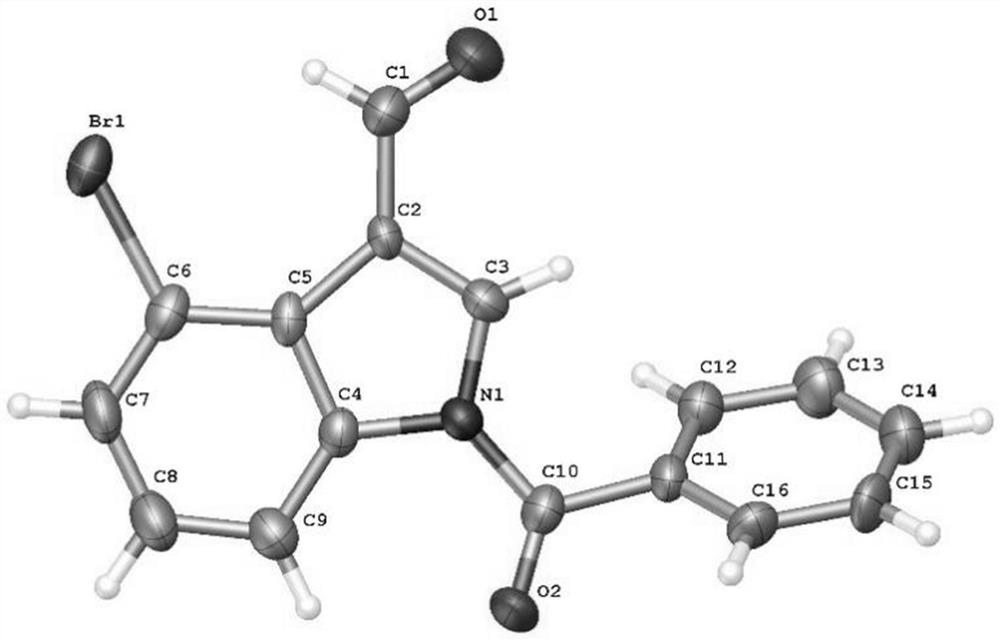
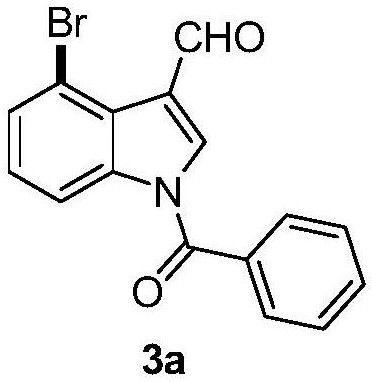
The present invention provides a synthetic method of Indiacens A, relates to the field of organic synthesis, comprising the following steps: (1) synthesis of 4-bromoindole-3-formaldehyde; (2) purification of 4-bromoindole-3-formaldehyde; (3) Indiacens A is synthesized from 4-bromoindole-3-formaldehyde. The present invention has the advantages of cheap raw materials, mature reaction, short synthesis steps and high yield.
Owner:DALI UNIV
A kind of fluorescent probe compound based on purine parent and its preparation method and application
ActiveCN112341463BHigh selectivityGood choiceOrganic chemistryFluorescence/phosphorescenceFluoProbesChemical compound
The invention discloses a fluorescent probe compound based on a purine parent, and also discloses a preparation method of the fluorescent probe compound and its application in detecting palladium ions and copper ions. In the present invention, a purine ring and 3-indole formaldehyde are used as fluorescent groups, and hydrazine hydrate is used as a linking group to synthesize a fluorescent probe compound based on a purine parent body, and the resulting fluorescent probe compound has high selectivity to copper palladium ions , high detection sensitivity, so it has the advantages of stable structure, good selectivity, high sensitivity and low toxicity; the preparation method of the invention has simple steps, easy-to-obtain raw materials, and the obtained product is solid powder, which is easy to store.
Owner:JIANGSU UNIV OF SCI & TECH
Preparation method and application of indole skeleton-containing chiral spiro compound catalyzed by nitrogen heterocyclic carbene
ActiveCN110551136BHigh enantioselectivityImprove universalityBiocideOrganic chemistry methodsCarbeneStructural formula
The present invention relates to the high enantioselectivity synthesis of indole skeleton-containing spiro compound (R)-1-aryl-1'H-spiro[dihydroindole-3,3'-spiro[dihydroindole-3,3'- The invention discloses a preparation method of oxazolo[3,4-α]indole]-1',2 diketone derivative and its good biological activity application. The general structural formula is as follows: where R 1 is a substituent for indole‑2‑carbaldehyde, R 2 is a substituent of indole-2,3-dione, R 3 are different protecting groups for indole‑2,3‑dione, methyl, benzyl, triphenylmethyl. Indole skeleton chiral spiro compound (R)-1-aryl-1'H-spiro[ Indoline-3,3'-oxazolo[3,4-α]indole]-1',2 dione derivatives, the derivatives of which have good universality and excellent yield up to 98%, Enantioselectivity up to 99% and good biological activity.
Owner:GUIZHOU UNIV
A kind of preparation method and application of indole-3-carboxaldehyde
ActiveCN108624633BLow costHigh purityOrganic chemistryMicroorganism based processesBiotechnologyChemical synthesis
The invention provides a method for preparing indole-3-formaldehyde, comprising the following steps: (1) inoculating Vibrio New Caledonia CGJ02-2 into LB liquid medium for cultivation, and centrifuging to remove bacteria after the cultivation body, harvest the fermentation supernatant; (2) extract the fermentation supernatant with an organic solvent, concentrate the organic phase under reduced pressure to obtain a crude extract; (3) separate the indole-3-formaldehyde from the obtained crude extract . In the present invention, the indole-3-formaldehyde is produced by a microbial fermentation method, and a large amount of indole-3-formaldehyde can be obtained only by cultivating the microorganisms with a simple culture medium. The method of the invention is not only low in cost, but also environmentally friendly than chemical synthesis. The in vitro enzyme activity test of indole-3-formaldehyde shows that it has strong xanthine oxidase inhibitory activity, and has great development potential and application prospects in the treatment of hyperuricemia and gout.
Owner:HAIKOU EXPERIMENTAL STATION CHINESE ACAD OF TROPICAL AGRI SCI
Chilled meat freshness markers and their screening and prediction model fitting methods and uses
ActiveCN111830181BAccurate detectionHigh resolutionComponent separationTesting foodBiotechnologyResolution (mass spectrometry)
The invention discloses a metabolomics-based chilled meat freshness marker and a screening and prediction model fitting method and application thereof. The markers include indole-3-formaldehyde, uridylic acid, phenylthiouric acid, gluconic acid, tyramine, serine-phenylalanine. The prediction model is: Y=3.964+1.97E ‑7 X 1 ‑4.22E ‑7 X 2 ‑3.37E ‑7 X 3 +8.80E ‑8 X 4 +1.26E ‑8 X 5 ‑5.57E ‑7 X 6 , This method uses Agilent 1290 UHPLC tandem Q Exactive Orbitrap high-resolution mass spectrometry, which has higher resolution, can detect more substances more accurately, and can more comprehensively elucidate the metabolism of chilled chicken during storage. product, the results obtained are more reliable.
Owner:YANGZHOU UNIV
A kind of indole vinyl substituted quinoline derivatives and its preparation method and application
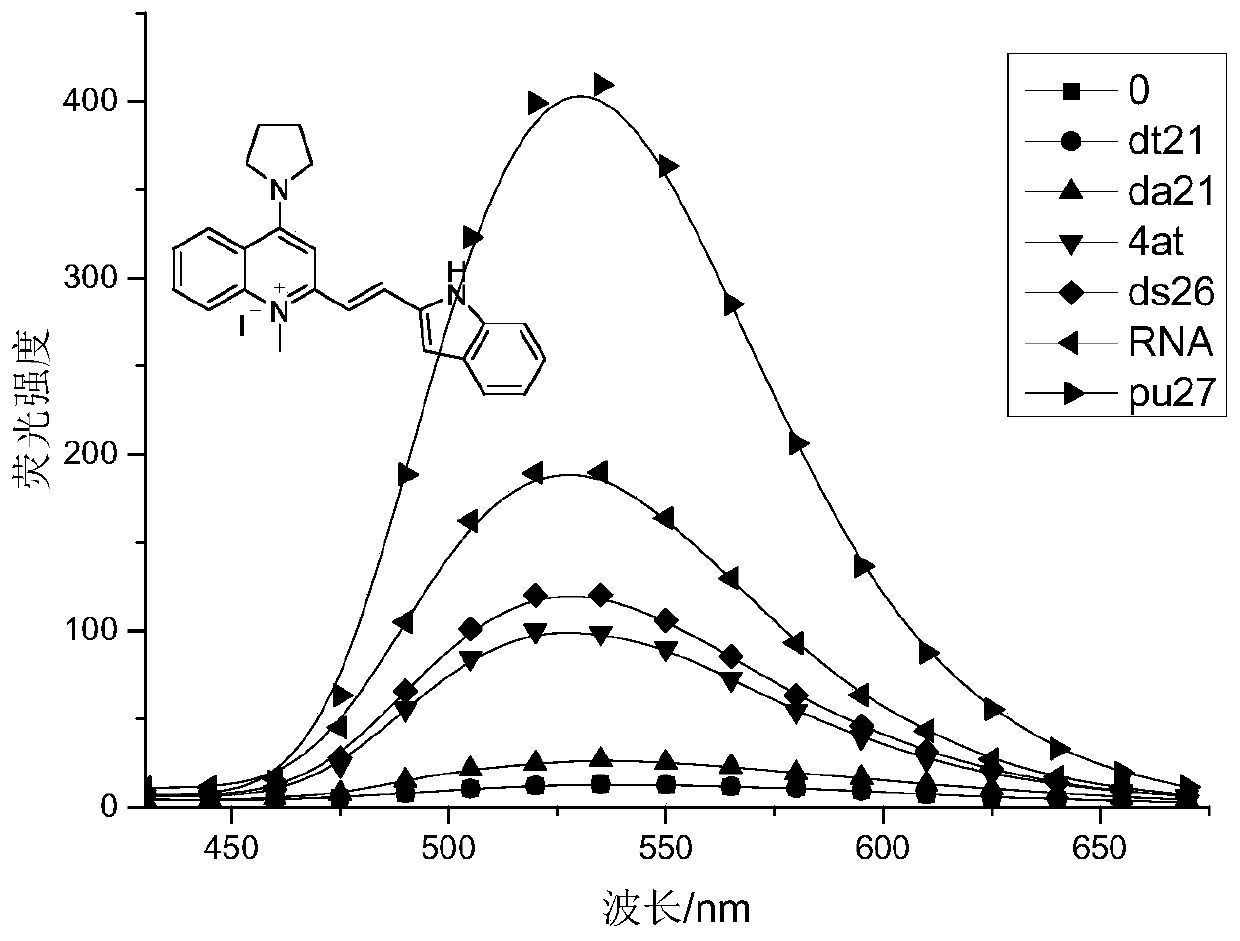
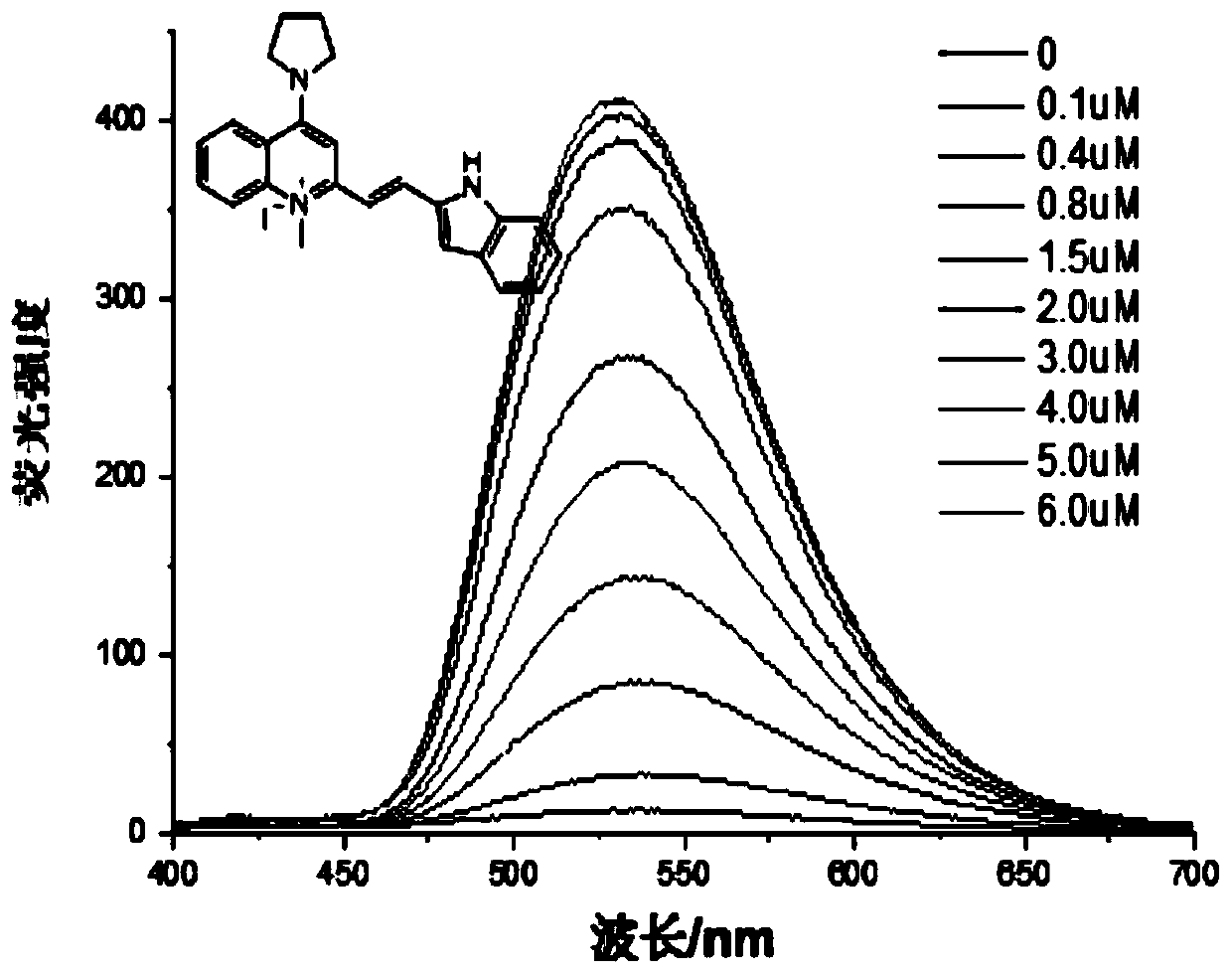
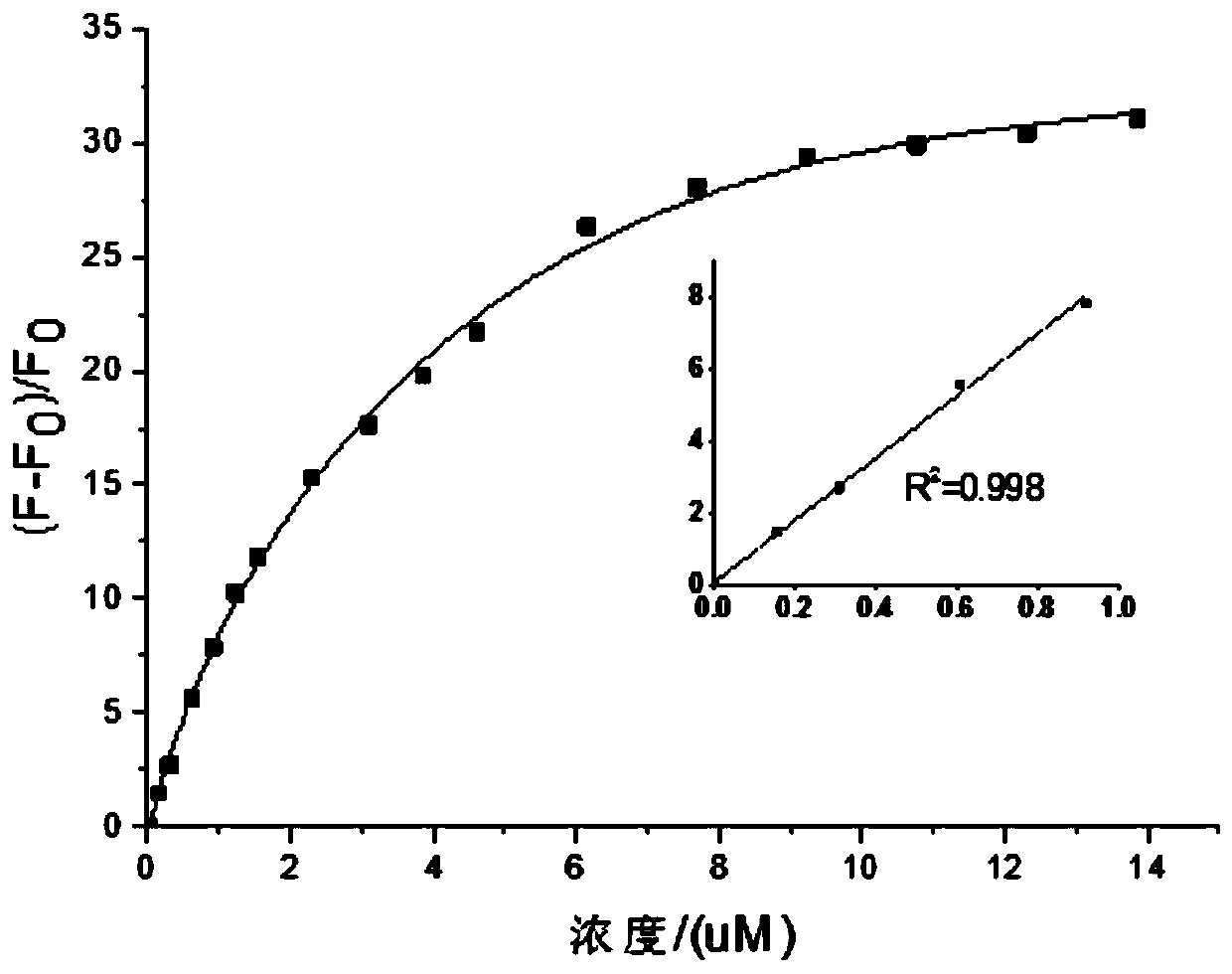
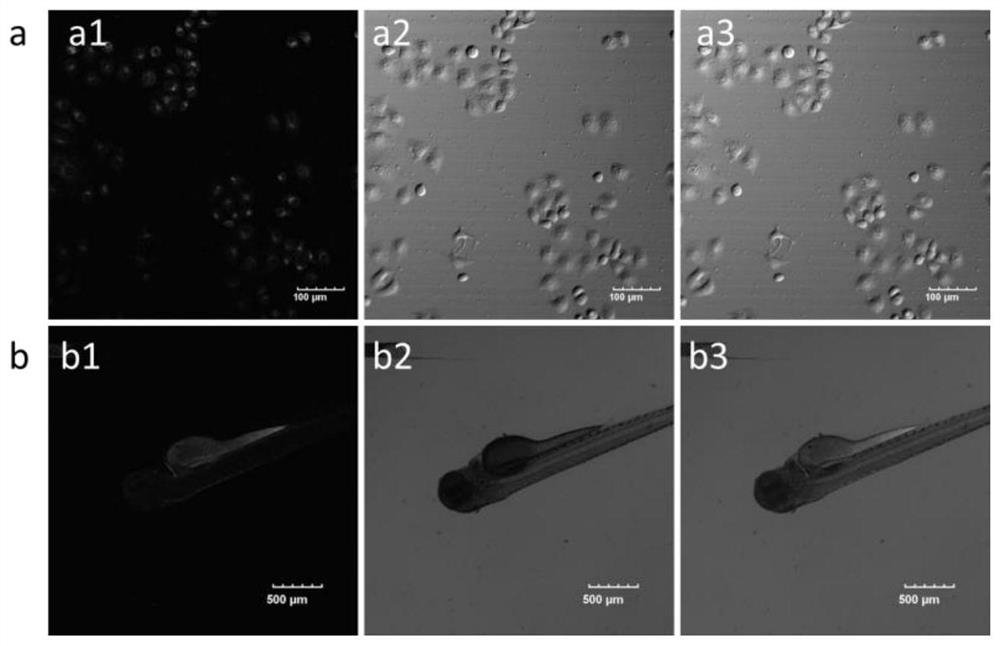
ActiveCN107266417BStrong forceNo signal changeOrganic chemistryFluorescence/phosphorescenceFluorescent spectraFluoProbes
Owner:GUANGDONG UNIV OF TECH
A kind of near-infrared two-photon photosensitizing dye sbopi and its preparation method and application
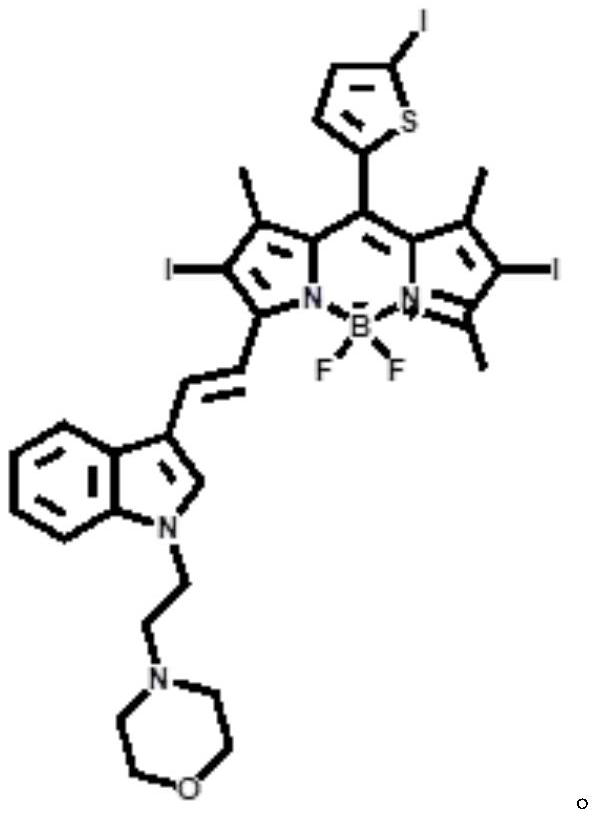
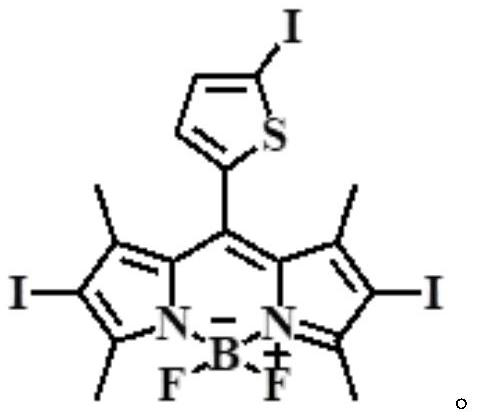
ActiveCN112552322BStrong two-photon absorption propertiesGood biocompatibilityMethine/polymethine dyesPhotodynamic therapyLysosomal targetingMorpholine
The invention discloses a near-infrared two-photon photosensitive dye SBOPI and its preparation method and application. The near-infrared two-photon photosensitive dye SBOPI comprises a push-pull electronic conjugated structure of thiophene-fluoroboron dipyrrole and indole and a lysosome target site. The preparation method of the near-infrared two-photon photosensitizing dye SBOPI is prepared by reacting iodothiophene-fluoroboridipyrrole SBOP and 1-(2-morpholinoethyl)-1H-indole-3-formaldehyde. The application of the near-infrared two-photon photosensitizing dye SBOPI is that under near-infrared light irradiation, the near-infrared two-photon photosensitizing dye SBOPI can efficiently release singlet oxygen, inhibit tumor cell migration, and promote tumor cell apoptosis. As a near-infrared two-photon photosensitizer and fluorescent dye with excellent performance, it has been widely used in near-infrared fluorescence imaging, fluorescence sensing, fluorescent labeling, and two-photon photodynamic therapy.
Owner:SOUTHEAST UNIV
Method for separating and purifying methyl p-hydroxybenzoate and 3-indolylformaldehyde from Trichosanthes kirilowii Maxim stem and leaf
InactiveCN103467297BSimple compositionExtended service lifeOrganic compound preparationCarboxylic acid esters separation/purificationBiotechnologyBenzoic acid
The invention relates to a method for separating and purifying methyl p-hydroxybenzoate and 3-indole formaldehyde from the stems and leaves of Trichosanthes chinensis. The stems and leaves of Trichosanthes chinensis are used as raw materials, and the following steps are carried out: (1) Crude extracts of stems and leaves of Trichosanthes chinensis (2) Extraction; (3) Separation and purification of reversed-phase C18 column; (4) Separation and purification of semi-preparative high-performance liquid chromatography: separation and purification by semi-preparative high-performance liquid chromatography, the mobile phase is methanol-water, Two high-purity components were obtained, which were identified as methyl p-hydroxybenzoate and 3-indole carboxaldehyde. The technological process is green and environmentally friendly, has no serious harm to the environment, and has low overall cost.
Owner:LIAOCHENG UNIV
4-bromoindole compound and its preparation method
ActiveCN112028812BHigh reaction yieldHigh chemoselectivityAntibacterial agentsNervous disorderSide reactionBiomedicine
The invention discloses a 4-position bromoindole compound and a preparation method thereof. Various N-protected indole-3-formaldehydes and N-bromosuccinimide (NBS) were used as reaction substrates to prepare 4-bromoindole compounds. The reaction yield can reach moderate to excellent, the reaction chemoselectivity and regioselectivity are excellent, the reaction conditions are mild, and the substrate has a wide range of application; it is easy to operate, low in cost, less in side reactions, high in product purity, and easy to separate Purification and can be applied to large-scale preparation, so the resulting product has a very good application prospect in the field of biomedicine.
Owner:PINGDINGSHAN UNIVERSITY +1
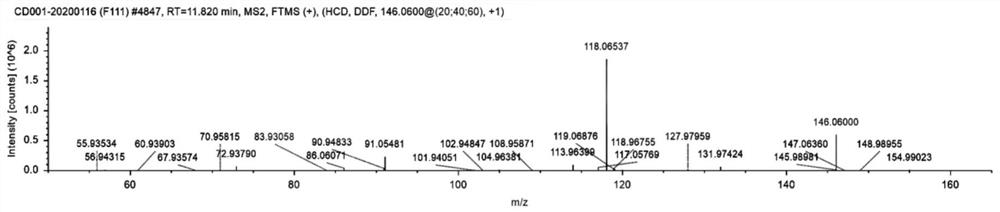
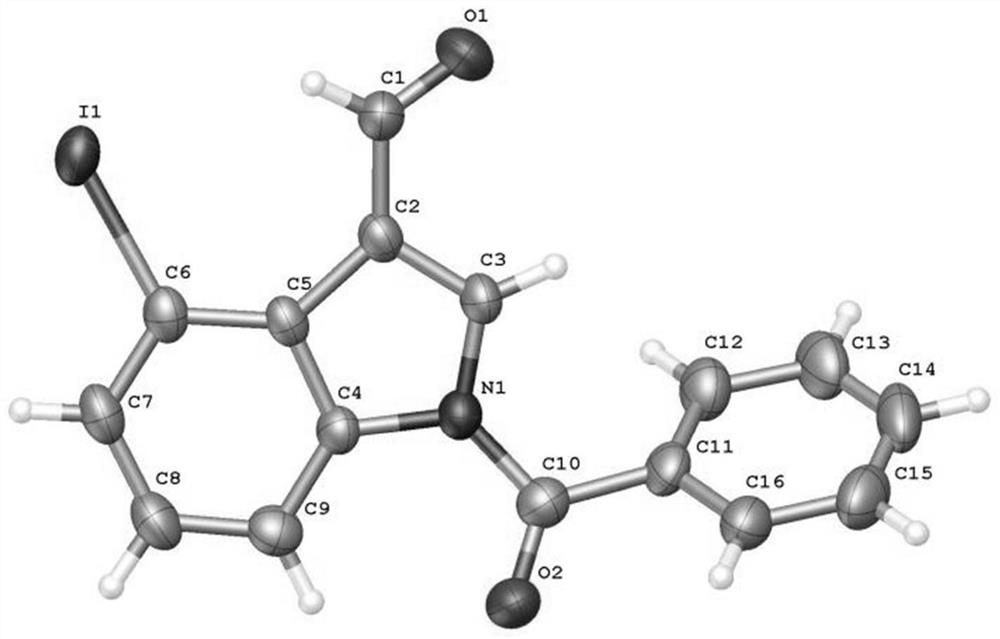
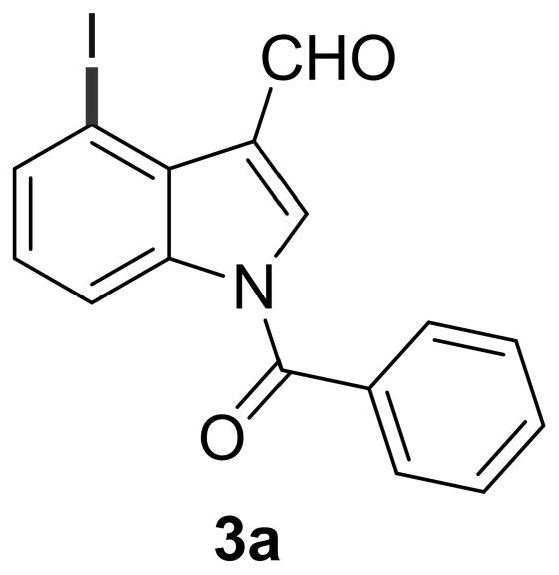
4-position iodoindole compound and preparation method thereof
ActiveCN112028811BHigh chemoselectivityHigh regional selectivityAntibacterial agentsNervous disorderImideHydrazine compound
The invention discloses a 4-position iodoindole compound and a preparation method thereof. Various N-protected indole-3-formaldehydes and N-iodosuccinimide (NIS) were used as reaction substrates to prepare 4-position iodoindole compounds. The reaction yield can reach moderate to excellent, the reaction chemoselectivity and regioselectivity are excellent, the reaction conditions are mild, and the substrate has a wide range of application; it is easy to operate, low in cost, less in side reactions, high in product purity, and easy to separate Purification and can be applied to large-scale preparation, so the resulting product has a very good application prospect in the field of biomedicine.
Owner:PINGDINGSHAN UNIVERSITY +1
A kind of indole-4-carbaldehyde compound with antibacterial activity in mulberry parasite, preparation method and application thereof
ActiveCN112321479BGood antibacterial effectEasy to prepareAntibacterial agentsOrganic active ingredientsOrganosolvStaphylococcus aureus
The invention discloses a compound with antibacterial activity in mulberry plants, a preparation method and its application in the preparation of antibacterial drugs, belonging to the field of phytochemistry. The present invention uses mulberry as raw material, dried and pulverized, soaked and extracted with an organic solvent, and then the extracts are combined, filtered and concentrated to obtain a mulberry extract; then extracted with n-butanol and methylene chloride, the The dichloromethane extract is concentrated under reduced pressure to obtain the dichloromethane extract extract; the dichloromethane extract extract is dissolved, mixed and then subjected to silica gel column chromatography, and the eluent is concentrated and then normal phase silica gel chromatography column (200 ~300 mesh) separation and purification, and gel column chromatography separation and purification to obtain the pure product of the target indole-4-formaldehyde. The product of the present invention has stronger antibacterial effect, and the minimum inhibitory concentration MIC value of described indole-4-formaldehyde compound to methicillin-resistant Staphylococcus aureus is 128 μ g / mL, and its antibacterial effect is far better than clindamylate hydrochloride Mycin (MIC=1024 μg / mL).
Owner:JILIN UNIV
4-iodo-indole compound and preparation method thereof
ActiveCN112028811ABiologically activePharmaceutically activeAntibacterial agentsNervous disorderImideRegioselectivity
The invention discloses a 4-iodo indole compound and a preparation method thereof. Various N-protected indolo-3-formaldehyde and N-iodosuccinimide (NIS) are used as reaction substrates to prepare the4-site iodoindole compound. The reaction yield can reach medium to excellent, the chemical selectivity and regioselectivity of the reaction are excellent, the reaction conditions are mild, and the application range of a substrate is wide. The method has the advantages of simple operation, low cost, few side reactions, high product purity, convenient separation and purification, and suitableness for large-scale preparation, so that the obtained product has a very good application prospect in the field of biological medicines.
Owner:PINGDINGSHAN UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com