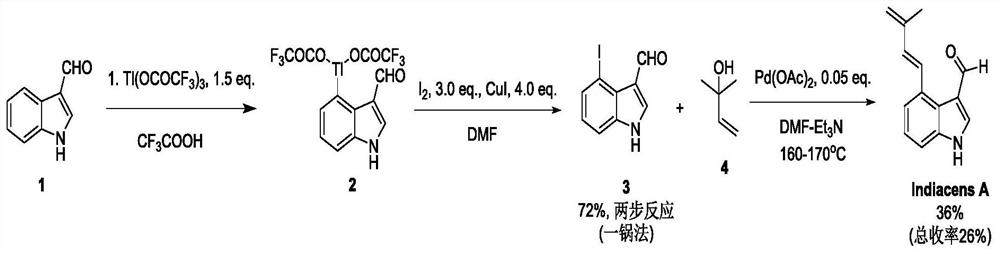
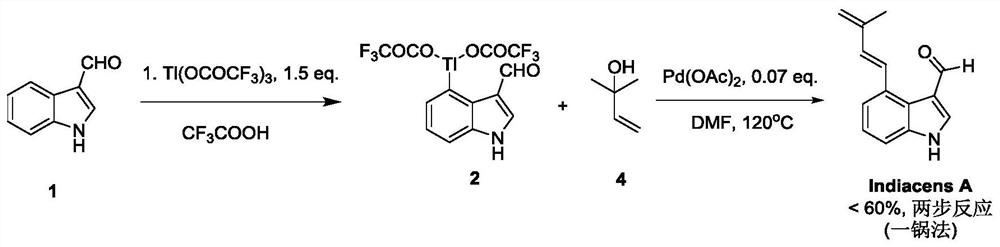
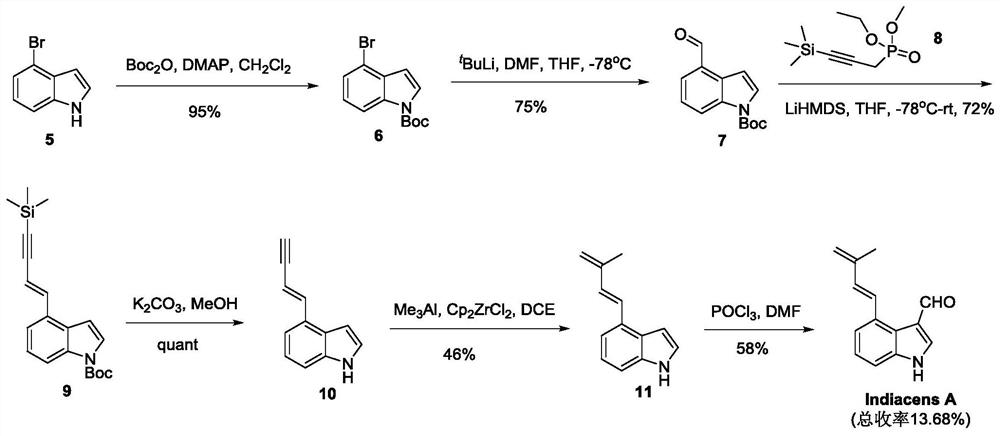
Synthesis of indiacens A
A synthesis method and dropping technique, applied in organic chemistry and other directions, can solve the problems of poor stability and increase the difficulty of the reaction, and achieve the effects of less solvent consumption, easy operation and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 120mL of dry N,N-dimethylformamide into a 500mL three-neck round bottom flask and cool down to 0°C, slowly drop in 11mL (0.12mol) of phosphorus oxychloride under vigorous stirring, continue stirring for 5min, slowly drop in Dissolve 10g (0.048mol) of 4-bromoindole (purity: 95%) in 20g of dry N,N-dimethylformamide. After the dropwise addition, remove the cold bath and continue the reaction for 1h. At this time, the reaction solution becomes viscous Slowly drop 4.7M potassium hydroxide solution into the reaction suspension (the dropping rate is to maintain the reaction solution at 65-75°C, if the temperature is too high, it can be cooled in a water bath), and the reaction is continued for 6 hours after the addition is completed. Slowly add 20 mL of a saturated solution of sodium bicarbonate dropwise, continue stirring for 5 min, add ethyl acetate to dilute and extract, dry, and concentrate under reduced pressure to obtain 12 g of the crude 4-bromoindole-3-carbaldehyde;...
Embodiment 2
[0034] Add 120mL of dry N,N-dimethylformamide into a 500mL three-neck round bottom flask and cool down to 0°C, slowly drop in 11mL (0.12mol) of phosphorus oxychloride under vigorous stirring, continue stirring for 10min, slowly drop in Dissolve 10g (0.048mol) of 4-bromoindole (purity 96%) in 20g of dry N,N-dimethylformamide. After the dropwise addition, remove the cold bath and continue the reaction for 2h. At this time, the reaction solution becomes viscous Slowly drop into the reaction suspension solution dissolved in 4.7M sodium hydroxide solution (the dropping rate is to maintain the reaction solution at 65-75°C, if the temperature is too high, it can be cooled in a water bath), and continue the reaction after the dropwise addition 8h, slowly add 20mL of saturated sodium bicarbonate solution dropwise, continue to stir for 10min, add ethyl acetate to dilute, extract, dry, and concentrate under reduced pressure to obtain 12g of the crude 4-bromoindole-3-carbaldehyde;
[0035...
Embodiment 3
[0038] Add 120mL of dry N,N-dimethylformamide into a 500mL three-neck round bottom flask and cool down to 0°C, slowly drop in 11mL (0.12mol) of phosphorus oxychloride under vigorous stirring, continue stirring for 15min, slowly drop in Dissolve 10g (0.048mol) of 4-bromoindole (purity: 97%) in 20g of dry N,N-dimethylformamide. After the dropwise addition, remove the cold bath and continue the reaction for 3h. At this time, the reaction solution becomes viscous Slowly drop into the reaction suspension solution dissolved in 4.7M lithium hydroxide solution (the dropping rate is to maintain the reaction solution at 65-75°C, if the temperature is too high, it can be cooled in a water bath), and continue the reaction after the dropwise addition 10h, slowly add 20mL of saturated sodium bicarbonate solution dropwise, continue stirring for 15min, add ethyl acetate to dilute, extract, dry, and concentrate under reduced pressure to obtain 13g of the crude 4-bromoindole-3-carbaldehyde;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com