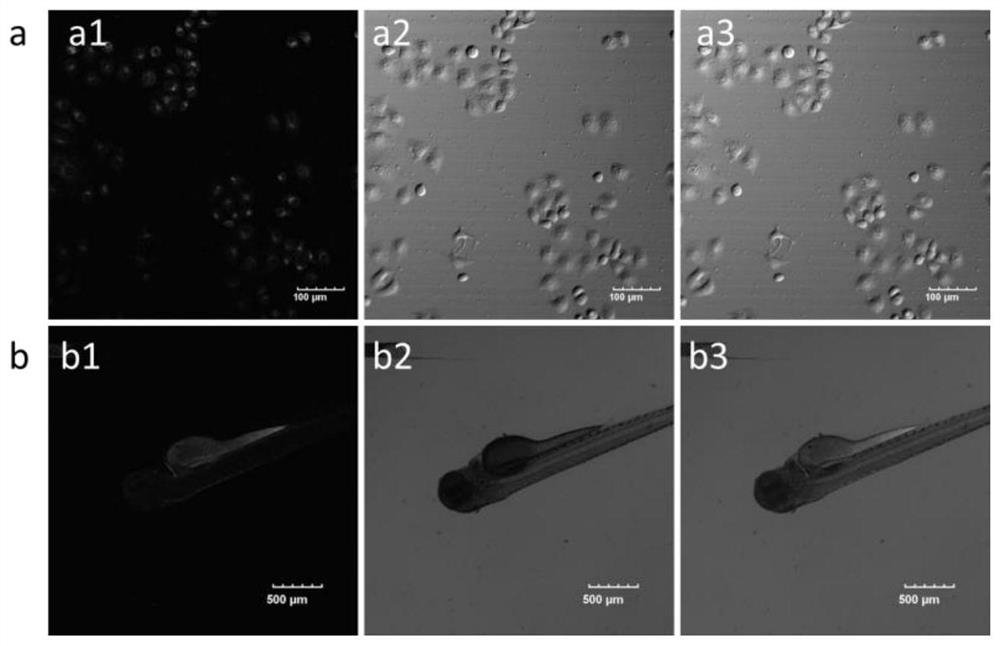
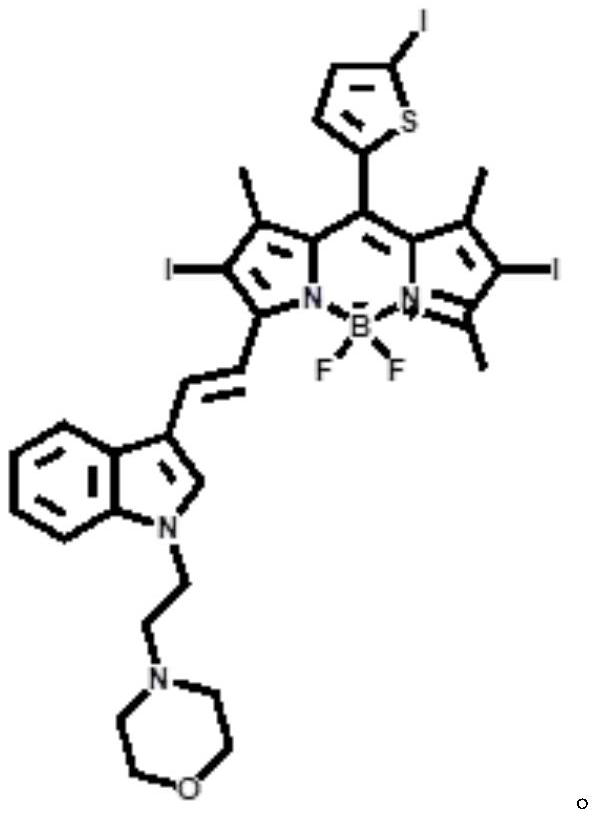
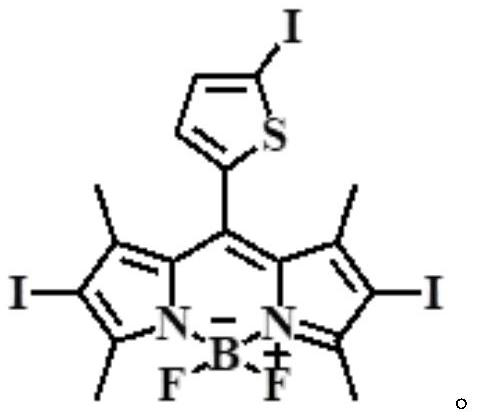
A kind of near-infrared two-photon photosensitizing dye sbopi and its preparation method and application
A photosensitizing dye and two-photon technology, applied in the field of photosensitizing dyes, can solve the problems of difficult to meet the photodynamic therapy of biological systems, low singlet oxygen yield, limited application scope, etc., so as to inhibit the migration of cancer cells and promote the apoptosis of tumor cells. , the effect of wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Preparation of near-infrared two-photon photosensitizing dye SBOPI:
[0022] Under nitrogen protection and 110°C, weigh 0.20 g of iodothiophene-fluoroboropipyrrole SBOP and dissolve it in 15 mL of dry toluene, add 0.09 g of 1-(2-morpholine ethyl)-1H-indole-3-carbaldehyde, Add 0.5 mL of glacial acetic acid dropwise, and stir for 6 h. After the reaction was completed, the photosensitizing dye SBOPI was obtained by vacuum distillation and column chromatography purification with a yield of 38%.
[0023] The synthetic route of photosensitizing dye SBOPI is as follows:
[0024]
[0025] Among them, iodothiophene-fluorobodipyrrole SBOP was prepared according to the literature method (Antoine Mirloup, Pascal Retailleau, Raymond Ziessel, Luminescent molecular solar concentrators made of multi-Bodipy dyes. Tetrahedron Letters, 2013, 54, 4456-4462).
[0026] (2) Structural characterization of near-infrared two-photon photosensitizing dye SBOPI:
[0027] H NMR spectrum 1...
Embodiment 2
[0032] The preparation of the near-infrared two-photon photosensitizing dye SBOPI is the same as in Example 1. Under nitrogen protection and 110°C, weigh 0.20 g of thiophene-fluoroboropipyrrole SBOP and dissolve it in 15 mL of dry toluene, add 0.11 g of 1-(2-morpholine ethyl Base)-1H-indole-3-carbaldehyde, then drop into 0.5mL glacial acetic acid, and stir for 6h. After the reaction, the near-infrared two-photon photosensitizing dye SBOPI was obtained by vacuum distillation and column chromatography purification, with a yield of 30.4%.
Embodiment 3
[0034] The preparation of the near-infrared two-photon photosensitizing dye molecule SBOPI is the same as in Example 1. Under nitrogen protection and 110°C, weigh 0.20g of thiophene-fluoroborate dipyrrole SBOP and dissolve it in 15mL of dry toluene, add 0.15g of 1-(2-morpholine Ethyl)-1H-indole-3-carbaldehyde, then dropwise into 0.5mL glacial acetic acid, and stirred for 6h. After the reaction, the near-infrared two-photon photosensitizing dye SBOPI was obtained by vacuum distillation and column chromatography purification, with a yield of 26.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com