A kind of preparation method and application of indole-3-carboxaldehyde
A formaldehyde and indole technology, applied in the field of medicine, can solve the problems of difficult separation of reactants and products, non-unique products, harsh experimental conditions, etc., and achieve huge development potential and application prospects, strong xanthine oxidase inhibitory activity, low cost cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of indole-3-carbaldehyde
[0034]1. Cultivation and fermentation of Vibrio New Caledonia
[0035] A single colony of Vibrio New Caledonia CGJ02-2 (deposited in China Center for Type Culture Collection (CCTCC) on December 18, 2017, deposit number: CCTCC M 2017802) was inoculated with an inoculation loop from a 2216E solid plate to Six 250 ml Erlenmeyer flasks each containing 100 ml of LB liquid medium containing 2% NaCl (g / ml) were cultured overnight at 28°C to 30°C and 160 rpm. The activated bacterial liquid was expanded and cultured with LB medium containing 2% NaCl at a ratio of 1:50, with a total volume of 16L, at 28°C-30°C, 120rpm for 10-12 days. The fermented liquid was centrifuged at 8000 rpm for 10 minutes in a high-speed centrifuge to remove the bacteria and harvest the supernatant.
[0036] 2. Extraction of fermentation broth
[0037] The culture supernatant was submerged in an equal volume of ethyl acetate for 24 hours, while shaking continuousl...
Embodiment 2
[0044] Determination of the Structure of Indole-3-Carboxaldehyde
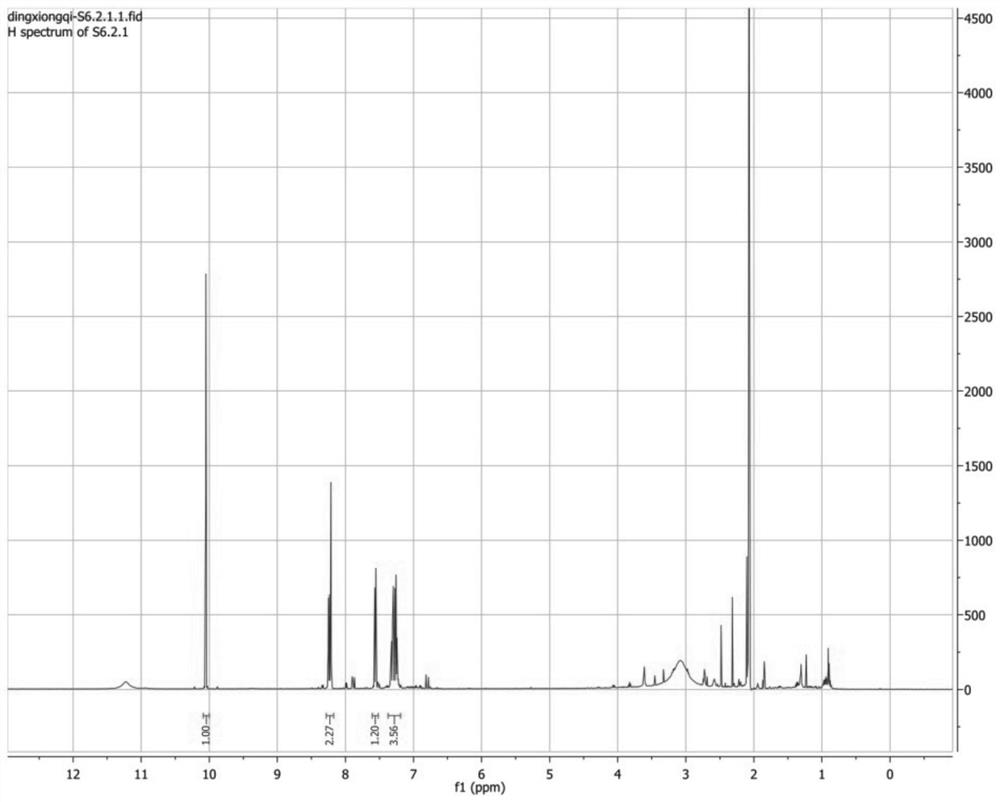
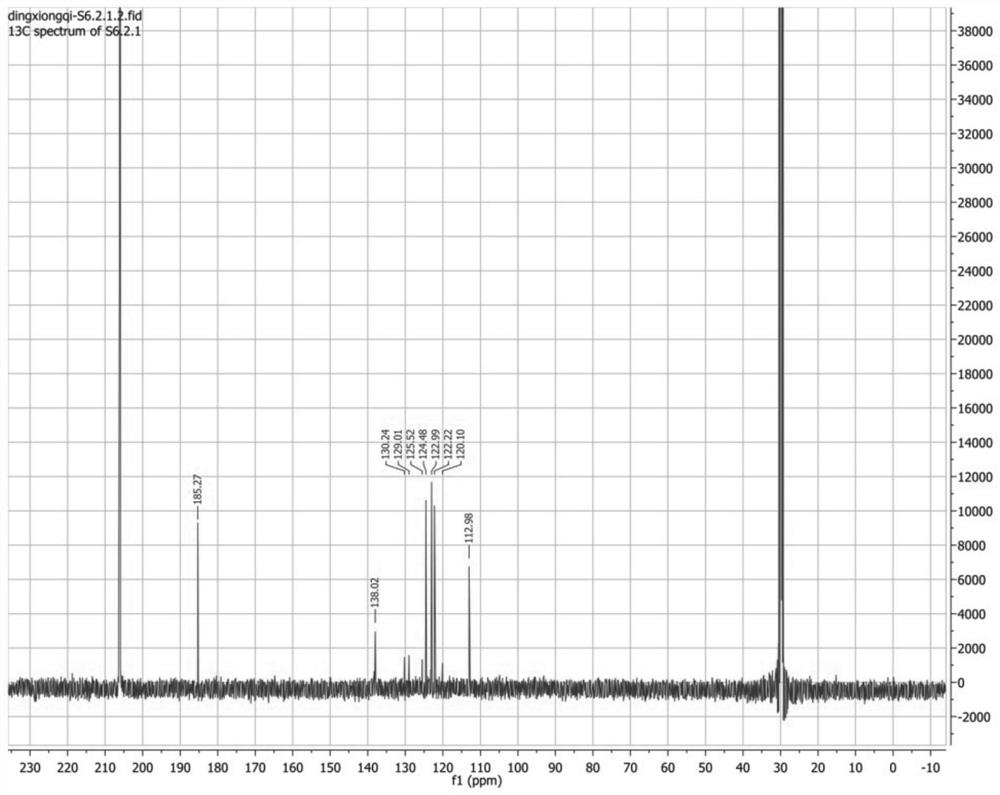
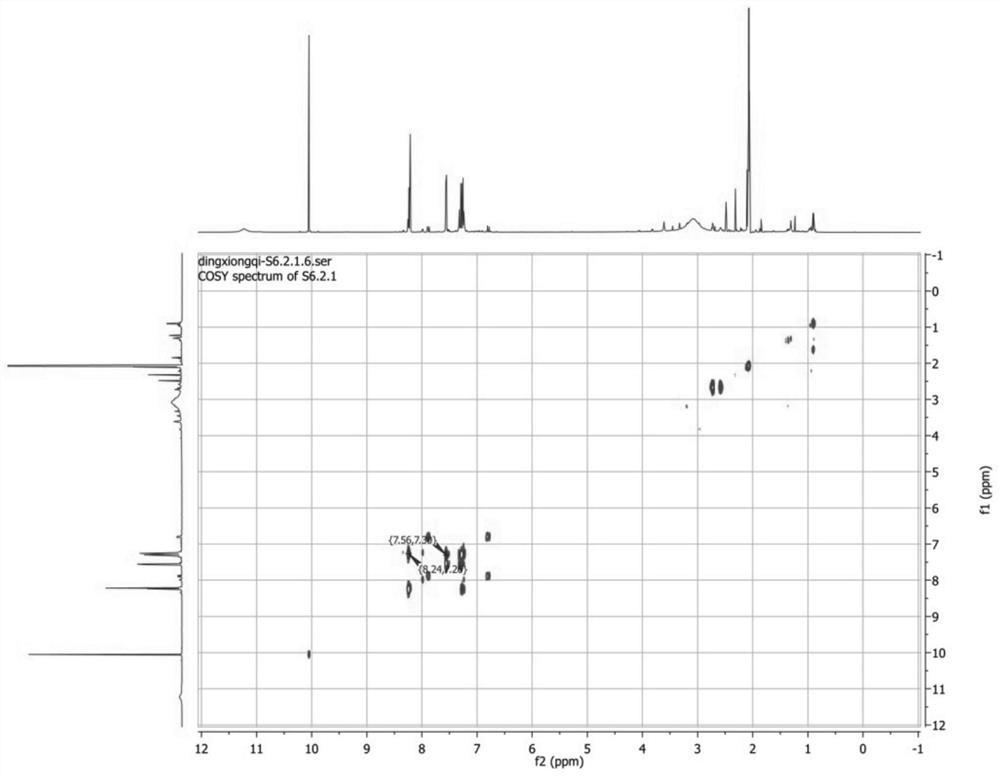
[0045] Take part F6.2.2.1 obtained in Example 1, and use 2D-NMR (nuclear magnetic resonance technique) (BRUKER AVANCE 500 MHz) for structure identification. in, figure 1 is the H-NMR spectrum, figure 2 for 13 C-NMR spectrum, image 3 is the COZY map, Figure 4 is the HMBC spectrum, Figure 5 It is the HSQC chart. Through structural analysis, the compound prepared by the present invention is indole-3-carboxaldehyde, and its chemical structural formula is:
[0046]
Embodiment 3
[0048] Determination of the inhibitory activity of indole-3-carbaldehyde on Xantine oxidase (XO)
[0049] Add 120 μL of 0.02 mol / L PBS (pH 7.4), 20 μL of different concentrations of indole-3-carbaldehyde, 40 μL of 1.5 mmol / L xanthine, and 20 μL of 0.5 U / mL xanthine oxidase in a 96-well plate , mixed thoroughly, and measured the absorbance at a wavelength of 295 nm with a full-wavelength microplate reader, using allopurinol as a positive control, and repeated 3 times to obtain the average value. Enzyme activity experiment is set as experimental group, experimental blank group, negative control, negative blank control, positive control and positive blank control 6 groups.
[0050] Experimental blank group: 40µl xanthine + 20µl sample + 120µl PBS + 20µl PBS
[0051] Negative control: 40µl xanthine + 20µl PBS + 120µl PBS + 20µl enzyme
[0052] Negative blank control: 40µl xanthine + 20µl PBS + 120µl PBS + 20µl PBS
[0053] Positive control: 40µl xanthine + 20µl allopurinol + 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com