Preparation method and application of indole skeleton-containing chiral spiro compound catalyzed by nitrogen heterocyclic carbene
A kind of technology of spiro compound and indole, which is applied in the field of preparation and application of chiral spiro compound containing indole skeleton catalyzed by nitrogen heterocyclic carbene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
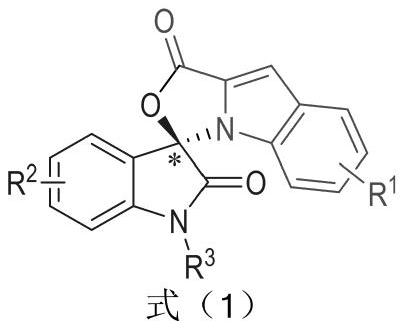
[0031] Substituent R 1 for H,R 2 for H, R 3 Be Bn, preparation method and condition are with general embodiment 1;
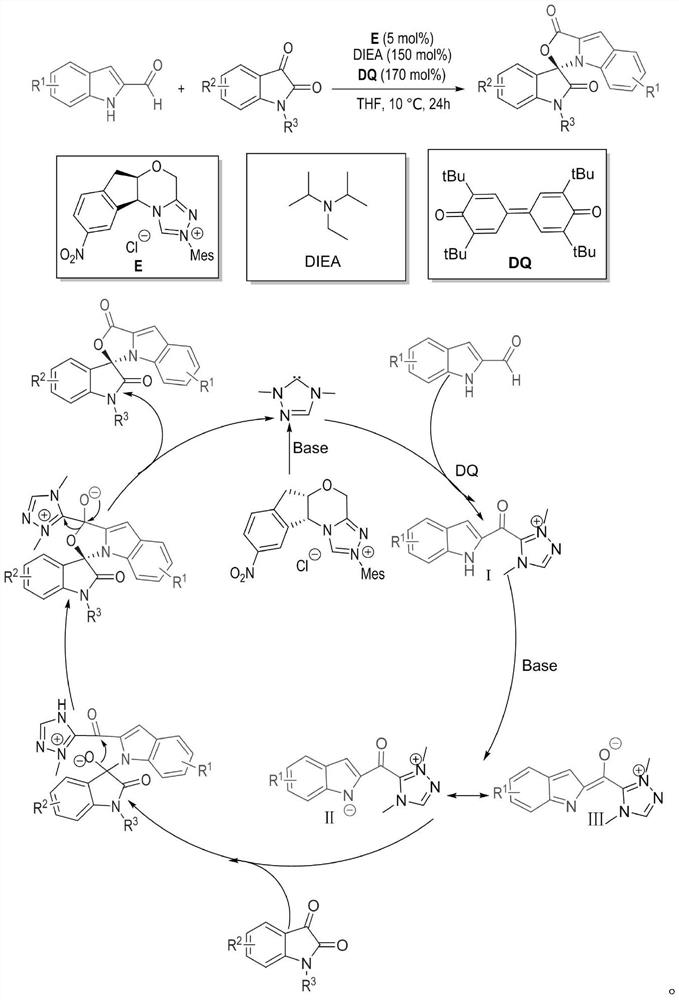
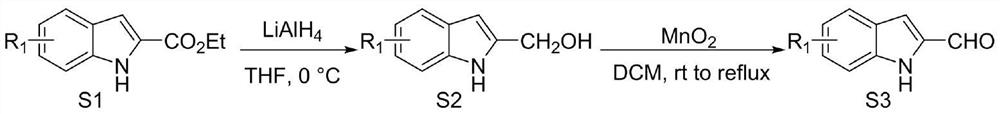
[0032] Weigh out 0.15mmol (23.73mg) indole-2-carbaldehyde 1, 0.1mmol (21.86mg) indole-2,3-dione 2, 0.05mmol (2.1mg) azacyclic carbene catalyst E and 0.17mmol respectively (69.5 mg) oxidant DQ was added to a 10 mL Schlenk reaction tube equipped with a magnetic stirrer bar, 1 mL solvent tetrahydrofuran THF was added, followed by 0.15 mmol (25 μL) base N,N-diisopropylethylamine DIEA, and the reaction wall was gently shaken to mix thoroughly. Cover the bottle and place it in an isopropanol water bath at 10°C to fully stir the reaction for 24h. After the reaction was monitored by TLC, 1 mL of 1N hydrochloric acid was added to the reaction tube, stirred at room temperature for 5 minutes, the organic layer was extracted with ethyl acetate, spin-dried, a small amount of dichloromethane was fully dissolved, and then loaded with wet method, separated by column chromat...
preparation Embodiment 2
[0039] Substituent R 1 as 4-Br,R 2 for H, R 3 Be Bn, preparation method and condition are with general embodiment 1;
[0040] (R)-1-Benzyl-8'-bromo-1'H-spiro[indoline-3,3'-oxazolo[3,4-α]indole]-1',2dione (I 2 )
[0041] 7.07–6.92 (m, 2H), 6.44 (d, J=8.4Hz, 1H), 5.11 (d, J=15.4Hz, 1H), 4.83 (d, J=15.4Hz, 1H);
[0042] 13 C NMR (101MHz, CDCl 3 )δ166.9,157.7,142.7,133.3,132.8,132.6,131.1,128.0,127.3,126.6,125.6,125.1,124.3,124.0,123.3,119.1,116.7,109.7,108.2,102.4,87.1;
[0043] HRMS (ESI,m / z)calcd.for C 24 H 15 N 2 O 3 Br H + :459.0338,found:459.0332;
[0044] Chiral analysis was performed by HPLC under the following conditions: 97:3er (U-IC column, 25°C, hexans / i PrOH=90 / 10, 0.3mL / min, λ=254nm), Rt(minor)=17.3min, Rt(major)=14.3min.
preparation Embodiment 3
[0046] Substituent R 1 for 4-OCH 3 ,R 2 for H, R 3 Be Bn, preparation method and condition are with general embodiment 1;
[0047] (R)-1-Benzyl-8'-methoxy-1'H-spiro[indoline-3,3'-oxazolo[3,4-α]indole]-1',2 diketone (I 3 )
[0048] 1H), 5.11(d, J=15.5Hz, 1H), 4.82(d, J=15.5Hz, 1H), 3.95(s, 3H);
[0049] 13 C NMR (101MHz, CDCl 3 )δ167.9,158.9,155.2,143.4,134.1,133.0,128.7,127.9,127.3,126.9,125.7,124.3,123.9,122.9,120.3,110.3,102.5,101.0,100.8,87.6,55.1
[0050] HRMS (ESI,m / z)calcd.for C 25 H 18 N 2 O 4 H + :411.1339,found:411.1329;
[0051] Chiral analysis was performed by HPLC under the following conditions: 97:3er (U-IC column, 25°C, hexans / i PrOH=90 / 10, 0.3mL / min, λ=254nm), Rt(minor)=41.9min, Rt(major)=32.9min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com