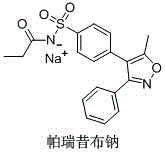
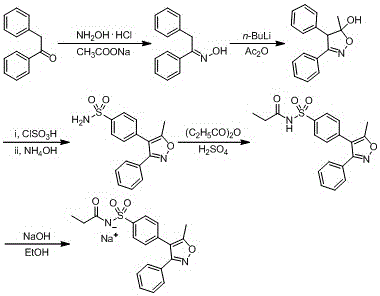
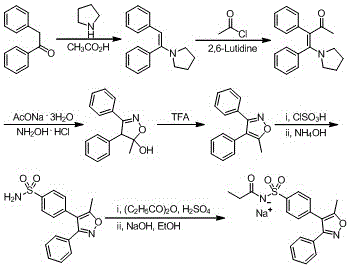
Synthesis method of parecoxib sodium
A technology for parecoxib sodium and a synthesis method, applied in the field of pharmaceutical production, can solve the problems of troublesome processing of the crude reaction product, little advantage in production, unfavorable production scale up and the like, and achieves the effects of short route steps, low cost and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step 1: Chlorosulfonation reaction
[0046]
[0047] Dissolve 98 g of chlorosulfonic acid in 75 mL of dichloromethane, cool to 5°C and add 5-methyl-3,4-diphenyl-isoxazole (25 g, from Shanghai Farmer Biotechnology Co., Ltd. purchased) in dichloromethane (50 mL); after the dropwise addition was completed, the system was heated up to reflux, and kept warm for reflux reaction for 8 hours. After the reaction was completed, it was cooled to room temperature, and the reaction system was slowly poured into 175 mL of ice water to quench the reaction, and the system was kept at 5°C. After the quenching reaction was complete, the liquids were left to separate, the aqueous phase was extracted with dichloromethane (62 mL X 2), the organic phases were combined, washed with water (190 mL X 3), and then concentrated to remove dichloromethane to obtain a concentrate. Add 250 mL of cyclohexane to the concentrate, heat to reflux for 30 minutes, add 75 mL of water, and continue to refl...
Embodiment 2
[0055] The difference with embodiment 1 is:
[0056] In Step 1, dissolve 75 g of chlorosulfonic acid in 60 mL of dichloromethane, cool to 3°C and add dropwise a solution of 5-methyl-3,4-diphenyl-isoxazole (25 g) in dichloromethane (50 mL); after the dropwise addition, the system was heated up to reflux, kept warm for reflux reaction for 7 hours; after the reaction was completed, cooled to room temperature, the reaction system was slowly poured into ice water to quench the reaction, and the system was kept at 3°C After the quenching reaction is complete, separate liquid, extract, wash, and concentrate to obtain a concentrate; add cyclohexane to the concentrate, heat and reflux for 20 minutes, add water, and continue to reflux for 20 minutes; separate liquid, remove the water phase, and leave the organic phase at room temperature Stir and crystallize for 30 minutes; obtain 20.2 g of intermediate 1 pure product, the purity is greater than 95%, and the yield is 58.7%, and its nucl...
Embodiment 3
[0060] The difference with embodiment 1 is:
[0061] In Step 1, dissolve 125 g of chlorosulfonic acid in 100 mL of dichloromethane, cool to 8°C and add dropwise a solution of 5-methyl-3,4-diphenyl-isoxazole (25 g) in dichloromethane ( 50 mL); after the dropwise addition, the system was heated up to reflux, and kept at reflux for 9 hours; after the reaction was completed, it was cooled to room temperature, and the reaction system was slowly poured into ice water to quench the reaction, and the system was maintained at 8°C; After the quenching reaction is complete, separate liquid, extract, wash, and concentrate to obtain a concentrate; add cyclohexane to the concentrate, heat to reflux for 50 minutes, add water, and continue to reflux for 50 minutes; separate liquid, remove the water phase, and stir the organic phase at room temperature Crystallization for 60 minutes; 20.8 g of intermediate 1 pure product was obtained, the purity was greater than 95%, and the yield was 59.2%. I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com