Preparation method of cinacalcet hydrochloride and intermediate thereof
A technology of cinacalcet hydrochloride and cinacalcet, which is applied in the field of preparation of cinacalcet hydrochloride and its intermediates, can solve the problems of unsuitability for industrial production, low product purity, long route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
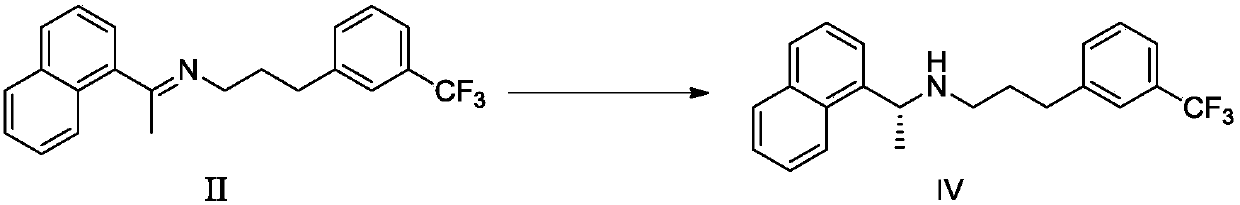
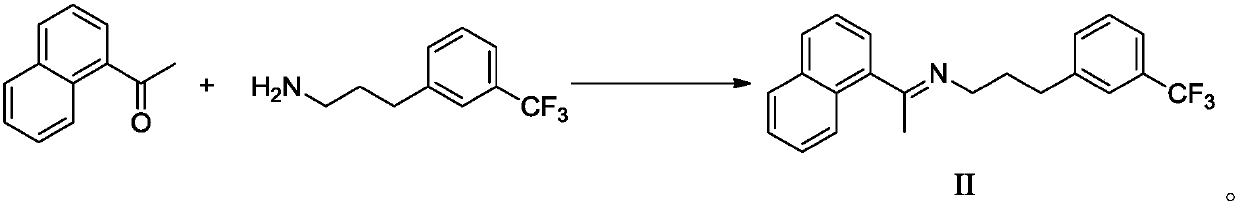
[0062] Example 1: Preparation of Cinacalcet Hydrochloride Intermediate II (refer to the method of Synthetic Route 1 in the specification of patent CN200980136709.1)
[0063]
[0064] Under the protection of nitrogen, add 100 g (0.587 mol) of 1-naphthyl ethyl ketone and 100 g (0.492 mol) of 3-trifluoromethylamphetamine into 700 mL of ethanol, then add 0.48 g (0.00499 mol) of methanesulfonic acid, and heat to 60-65 Stir at ℃ for 3-4 hours, then concentrate in vacuo (-0.085MPa--0.1MPa, 45-55℃) to remove the solvent. Add 350mL of n-heptane, stir at 15-20°C for 1-2 hours, discard the supernatant after standing and repeat three times; vacuum concentration (-0.085MPa~-0.1MPa, 45-55°C) to obtain cinacalcet Intermediate II 161.0g, yield 92.05%, HPLC purity 98.37%.
Embodiment 2
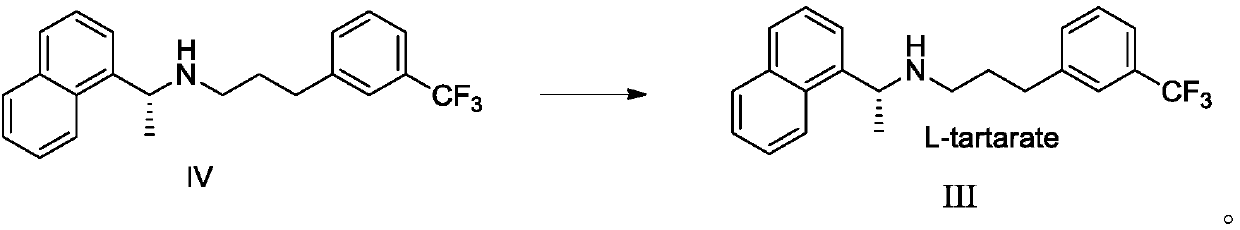
[0065] Embodiment 2: the preparation of L-cinacalcet III tartrate
[0066]
[0067] Add 20 g (0.0563 mol) of cinacalcet intermediate II, 27 mg (0.0000576 mol) of bis(1,5-cyclooctadiene)-rhodium trifluoromethanesulfonate, (S)-3,3' to the hydrogenation kettle - Bis(2,4,6-triisopropylphenyl)-1,1'-di-2-naphthol cyclic phosphate (L*) 44 mg (0.0000584 mol) and ethanol 100 mL. Hydrogenation is carried out at a hydrogen pressure of 4-6 atmospheres and 40-50° C. for 2.5-3.5 hours.
[0068] Cool, filter, wash with ethanol and concentrate in vacuo (45°C~55°C, -0.085MPa~-0.1MPa) to remove the solvent to obtain crude cinacalcet IV. Then, 70 mL of isopropyl acetate was added, stirred and filtered, and a solution of 8.45 g of L-tartaric acid in 70 mL of ethanol was added to the filtrate. Heat to 60℃~65℃ and stir for 0.5 hours, cool to 5℃~10℃ and stir for 1~2 hours, filter, wash once with isopropyl acetate, vacuum dry (45℃~55℃, -0.01MPa~-0.1MPa ) in 12 to 16 hours to obtain an off-white...
Embodiment 3
[0069] Embodiment 3: the preparation of L-cinacalcet III tartrate
[0070]
[0071] Add 60 g (0.169 mol) of cinacalcet intermediate II, 40 mg (0.0000854 mol) of bis(1,5-cyclooctadiene)-rhodium trifluoromethanesulfonate, (S)-3,3' to the hydrogenation kettle - Bis(2,4,6-triisopropylphenyl)-1,1'-di-2-naphthol cyclic phosphate (L*) 65 mg (0.0000863 mol) and ethanol 180 mL. Hydrogenation was carried out at a hydrogen pressure of 6-8 atmospheres and 30-40° C. for 3.5-4.5 hours.
[0072] Cool, filter, wash with ethanol and concentrate in vacuo (45°C~55°C, -0.085MPa~-0.1MPa) to remove the solvent to obtain crude cinacalcet IV. Add 210 mL of isopropyl acetate, stir and filter, then add a solution of 25.4 g of L-tartaric acid in 210 mL of ethanol to the filtrate. Heat to 60℃~65℃ and stir for 0.5 hours, cool to 5℃~10℃ and stir for 1~2 hours, filter, wash once with isopropyl acetate, vacuum dry (45℃~55℃, -0.01MPa~-0.1MPa ) in 12 to 16 hours to obtain an off-white solid which was L-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com