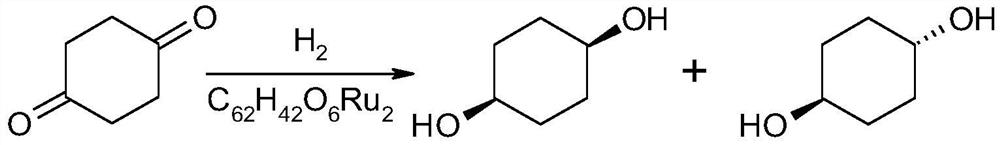
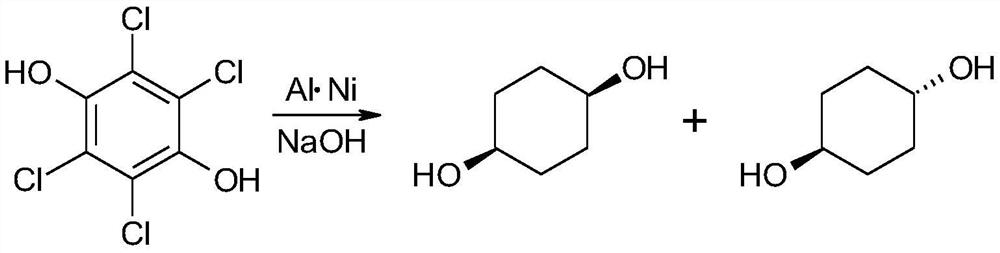
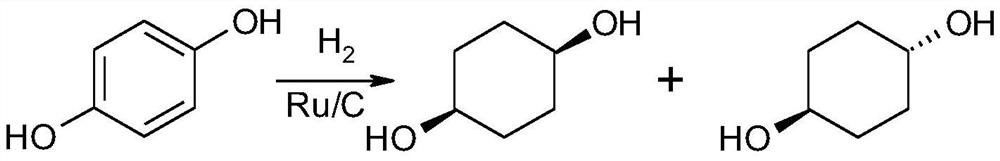
A kind of synthetic method of cis-1,4-cyclohexanediol
A synthesis method and compound technology, applied in the directions of organic chemistry methods, chemical instruments and methods, formation/introduction of hydroxyl groups, etc., can solve the problems of unsuitable promotion and rare raw materials, and achieve the effects of short steps, convenient purification and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] 1. Preparation of Compound III-1 from Compound II-1
[0037] Dissolve raw material II-1 (148.0g, 0.641mol, 1.0eq.) in methanol (1.2L), add KBH in batches at -15°C 4 (68.8g, 1.28mol, 2.0eq.), after the addition was complete, the reaction was maintained at this temperature for 4 hours.
[0038] Add 1.5 L of saturated ammonium chloride solution dropwise to the reaction mixture, stir until no bubbles are generated, add ethyl acetate for extraction, combine the organic phases for column chromatography (petroleum ether: ethyl acetate is 10:2), and remove under reduced pressure After solvent, white solid III-1 (68.5g, 0.30mol) yield: 46.1%; and white solid IV-1 (45.7g, 0.197mol) were obtained, yield: 30.8%. 1 H NMR (400M Hz, DMSO-d 6 )δ(ppm)7.24-7.27(m,5H),4.65(m,1H),3.75(s,2H),3.58(br,1H),1.57-1.54(m,4H),1.44-1.40(m, 4H).
[0039] 2. Preparation of Compound I from Compound III-1
[0040] Dissolve III-1 (68.5g, 0.30mol, 1.0eq.) in 90% aqueous methanol (0.8L), ...
Embodiment 2
[0042]
[0043] 1. Preparation of Compound III-2 from Compound II-2
[0044] The material II-2 (156.0g, 0.788mol, 1.0eq.) was dissolved in ethanol (1.5L), and NaBH was added in batches at 10°C 4 (53.7g, 1.42mol, 1.8eq.), after the addition was complete, the reaction was maintained at this temperature for 1.5 hours.
[0045] Add 2.0L of saturated sodium chloride solution dropwise to the reaction mixture, stir until no bubbles are generated, add ethyl acetate for extraction, combine the organic phases for column chromatography (petroleum ether:ethyl acetate:ethanol is 20:3:1), After removing the solvent under reduced pressure, white solid III-2 (75.8 g, 0.381 mol) yield: 48.3%; and white solid IV-2 (41.7 g, 0.209 mol) were obtained, yield: 26.6%. 1 H NMR (400M Hz, DMSO-d 6 )δ (ppm) 4.61 (m, 1H), 4.29-4.28 (d, 1H), 1.57-1.54 (m, 4H), 1.44-1.40 (m, 4H), 1.27 (s, 9H).
[0046] 2. Preparation of Compound I from Compound III-2
[0047]Dissolve III-2 (75.8g, 0.381mol, 1.0eq.) i...
Embodiment 3
[0049]
[0050] 1. Preparation of Compound III-3 from Compound II-3
[0051] Material II-3 (176.5g, 1.13mol, 1.0eq.) was dissolved in methanol (2.5L), and LiBH was added in batches at -10°C 4 (36.9g, 1.70mol, 1.5eq.), after the addition was complete, the reaction was maintained at this temperature for 3.5 hours.
[0052] Add 3.0L saturated sodium chloride solution dropwise to the reaction mixture, stir until no bubbles are generated, add ethyl acetate for extraction, combine the organic phases for column chromatography (petroleum ether: methylene chloride 5:1), remove under reduced pressure After solvent, white solid III-3 (82.7g, 0.526mol) yield: 46.6%; and white solid IV-3 (45.0g, 0.286mol) were obtained, yield: 25.3%. 1 H NMR (400M Hz, DMSO-d 6 )δ (ppm) 4.59 (m, 1H), 4.29-4.28 (d, 1H), 2.02 (s, 3H), 1.57-1.54 (m, 4H), 1.44-1.40 (m, 4H).
[0053] 2. Preparation of Compound I from Compound III-3
[0054] Dissolve III-3 (82.7g, 0.526mol, 1.0eq.) in 90% aqueous methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com