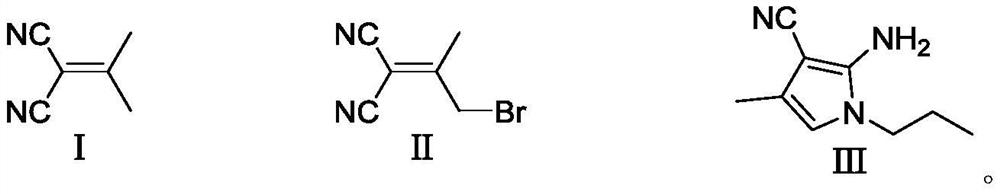
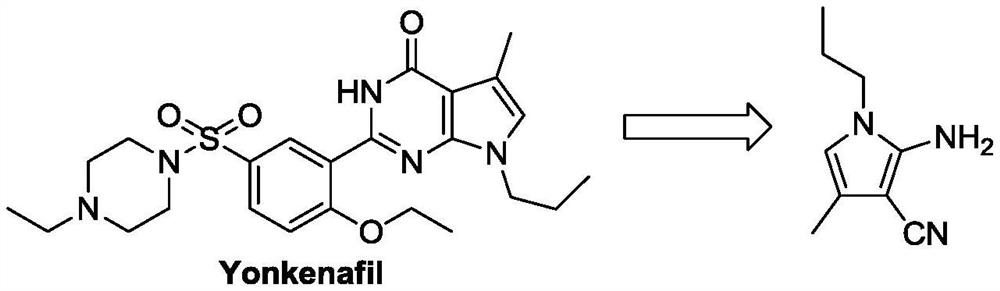
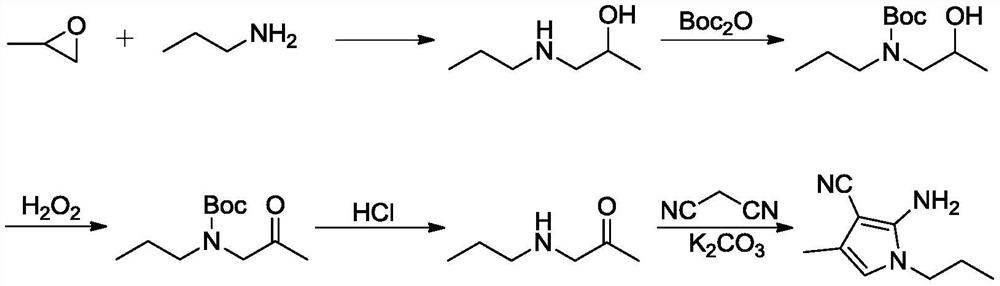
Preparation method of 4-methyl-1-propyl-2-amino-1H-pyrrole-3-nitrile
A technology of propyl and amino groups, applied in the field of organic synthesis, can solve the problems of unfavorable industrial production, risk of production enlargement, long reaction steps, etc., and achieve the effects of short steps, reduction of three wastes, and short route steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Add malononitrile (11.67g, 0.177mol), acetone (13mL, 0.177mol) and chloroform (100mL) into the reaction flask, and slowly add aluminum oxide (35g). After the dropwise addition, react at 20-25°C for 1h. After the reaction was completed, it was filtered, extracted with chloroform (2*50 mL), and concentrated to obtain 17 g of the compound of formula (I) (yield 100%).
Embodiment 2
[0049]
[0050] Add intermediate formula (I) compound (30g, 0.28mol), NBS (65g, 0.37mol), benzoyl peroxide (0.6g, 2.48mmol) and chloroform (90mL) into the reaction flask, heat up to 60-70°C Reflux reaction for 15h. After the reaction was completed, it was cooled, filtered, washed with chloroform, and the filtrate was concentrated and then rectified to obtain 36.6 g of the compound of formula (II) (yield 70%).
Embodiment 3
[0052]
[0053] Add the compound of formula (II) (13.1g, 62mmol), n-propylamine (5.5g, 93mmol), ethanol (100mL) into the reaction flask, and react at 20-30°C for 6h. After the reaction is completed in LC, dry it under reduced pressure Ethanol, 200mL of water and 200mL of ethyl acetate were added, the organic phase was separated and dried to obtain a crude product, the crude product was salted with HCl / EA, the precipitated product was filtered, and 10.4g of product formula (Ⅲ) compound was obtained after freeing (90% yield). 1 H NMR(400MHz,DMSO)δ0.801-0.838(m,3H),1.534-1.589(m,2H),1.916-1.919(s,3H),3.553-3.589(m,2H),5.626(s,2H ), 5.955-5.958(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com