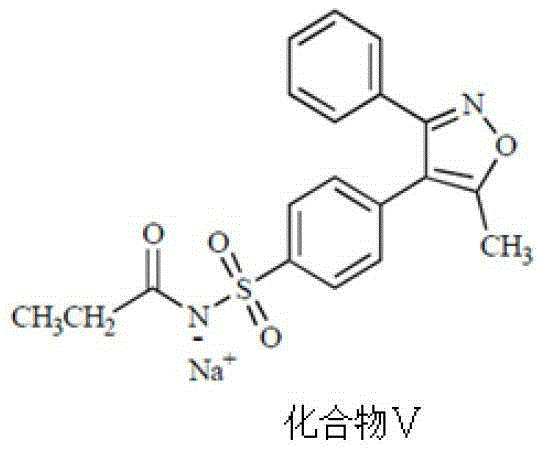
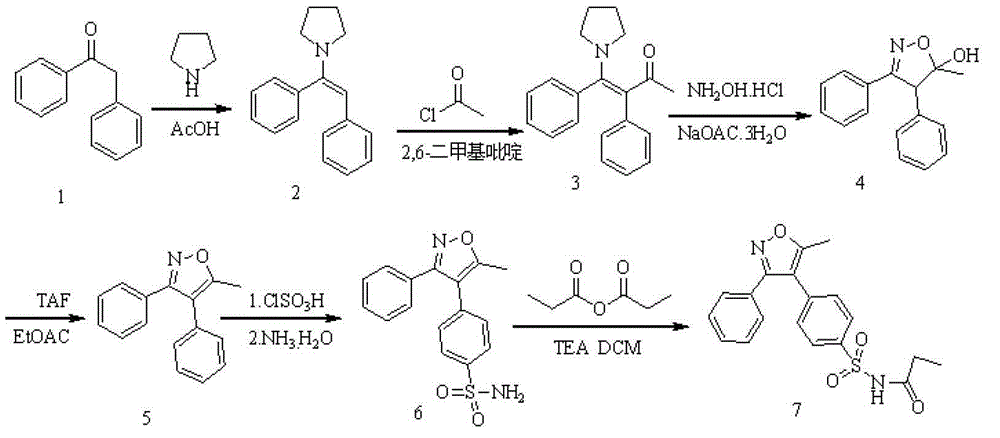
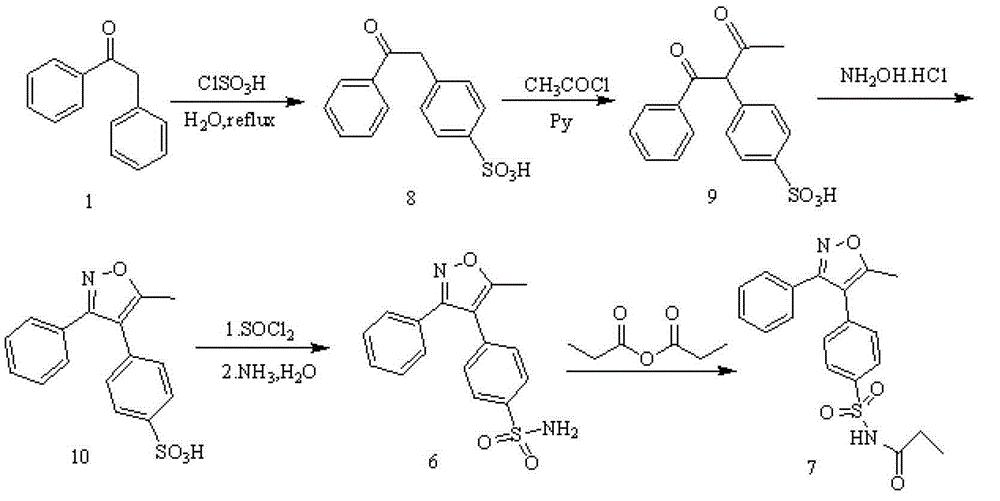
Preparing method for parecoxib sodium
A technology of parecoxib sodium and its compound, which is applied in the field of preparation of parecoxib sodium, can solve the problems of low yield, long oxazole ring process route, unsuitability for industrial production, etc., achieve high selectivity and shorten synthesis The process route is conducive to the effect of enlarging production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of compound III
[0048] First add 12.11g (100mmol) of benzaldoxime (Compound I) into 200ml of dichloromethane, then add 16.02g (120mmol) of N-chlorosuccinimide (NCS), keep the temperature for 5 hours, then add 1- Add 6.97g (60mmol) of phenyl-1-propyne (compound II), add 12.12g (120mmol) of triethylamine, react at room temperature, monitor the reaction by thin layer chromatography, stop the reaction after the reaction is complete. The reaction solution was washed successively with dilute hydrochloric acid and saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and recrystallized from a mixture of petroleum ether and ethyl acetate to obtain 12.74 g (54.2 mmol) of compound III with a yield of 90.3 %, purity 99.5% (HPLC method).
Embodiment 2
[0049] Embodiment 2: the synthesis of compound III
[0050] First, add 24.23g (200mmol) of benzaldoxime (compound I) into 500ml of dichloromethane, add 40.06g (300mmol) of N-chlorosuccinimide (NCS), maintain the temperature for 3 hours, then add 1- Phenyl-1-propyne (Compound II) 11.62g (100mmol) was added with 30.31g (300mmol) of triethylamine, the temperature was controlled at 60°C, and the reaction was monitored by TLC. After the reaction was complete, the reaction was stopped. The reaction solution was washed successively with dilute hydrochloric acid and saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and recrystallized from a mixture of petroleum ether and ethyl acetate to obtain 21.75 g (92.5 mmol) of compound III with a yield of 92.5 %, purity 99.2% (HPLC method).
Embodiment 3
[0051] Embodiment 3: the synthesis of compound III
[0052] First, add 12.11g (100mmol) of benzaldoxime (compound I) into 300ml of methyl chloride, then add 13.35g (100mmol) of N-chlorosuccinimide (NCS), keep the temperature for 2 hours, then add 1-benzene Diethylamine 10.10 g (100 mmol) was added to 11.62 g (100 mmol) of 1-propyne (compound II), and the temperature was controlled at 80° C. to react, and the reaction was monitored by thin-layer chromatography. After the reaction was complete, the reaction was stopped. The reaction solution was washed successively with dilute hydrochloric acid and saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and recrystallized from a mixture of petroleum ether and ethyl acetate to obtain 21.58 g (91.8 mmol) of compound III, with a yield of 91.8 %, purity 99.1% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com