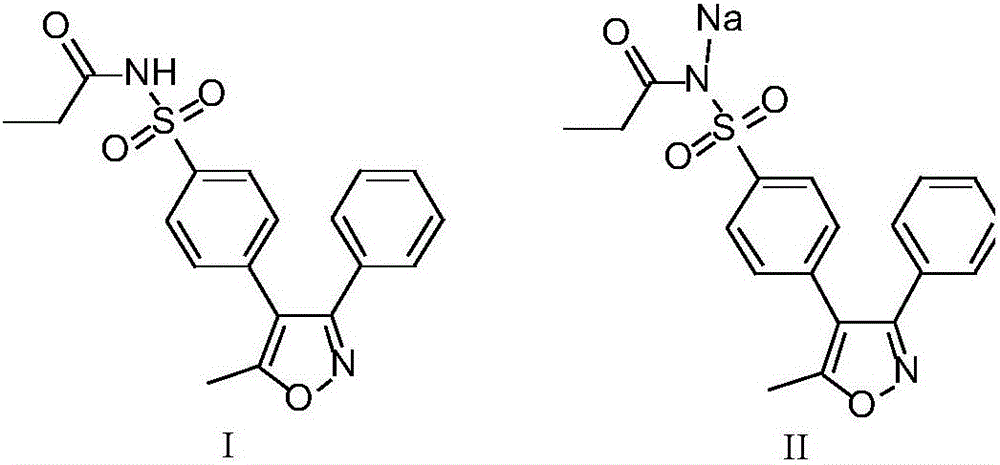
Preparation methods of parecoxib sodium and intermediate thereof
A technique for parecoxib sodium and intermediates, which is applied in the field of preparation of parecoxib sodium and its intermediates, can solve the problems of unsuitability for industrial production, low reaction yield, high production cost, etc., and achieve low cost and high purity High, responsive and easy post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
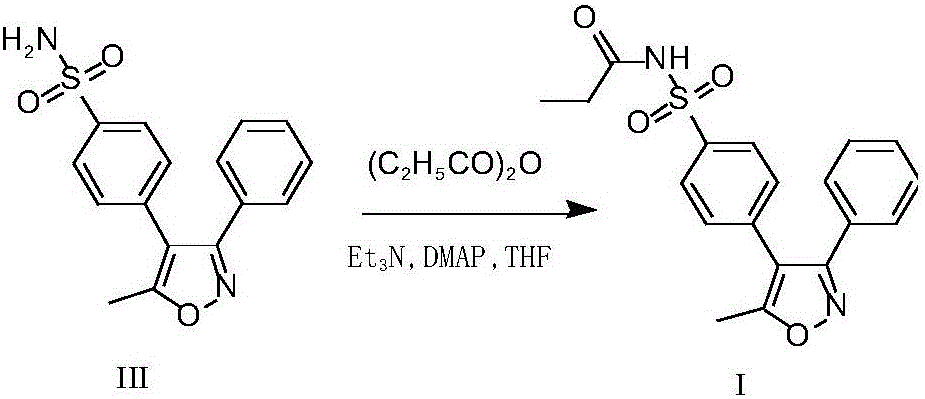
[0038] Embodiment 1: the preparation method of parecoxib sodium intermediate I
[0039]
[0040] The raw material valdecoxib III (5.0 g) was added to propionic anhydride (18.6 g), and stirred to dissolve. Add sulfamic acid (0.15g), heat up to 50-60°C, and react for about 3 hours. TLC monitors that the reaction is complete, cools down to about 0°C, and keeps stirring for about 1 hour. Filter, rinse the filter cake with methyl tert-butyl ether (10ml) and place it in a vacuum oven at -0.08MPa~-0.1MPa, and dry it in vacuum at 50°C for 8-12 hours to obtain 4.77g of the intermediate of parecoxib sodium 1, yield 81%. The HPLC purity is 99.91%, the maximum impurity is 0.06%, and the raw material valdecoxib III is <0.02%.
Embodiment 2
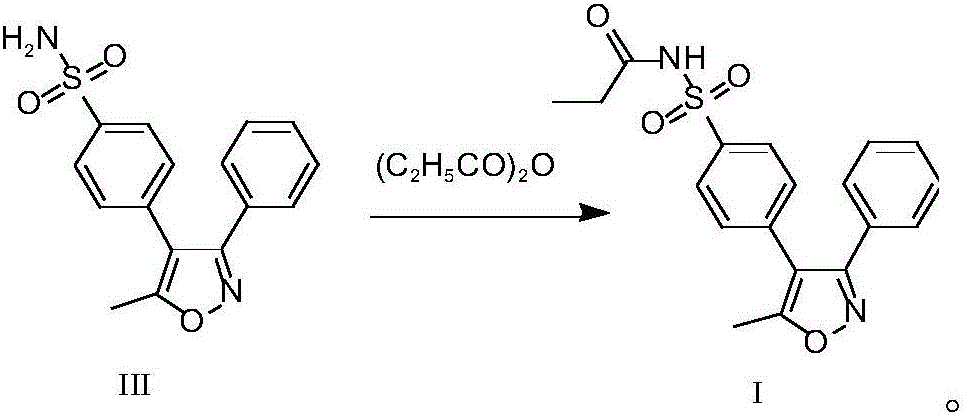
[0041] Embodiment 2: the preparation method of parecoxib sodium intermediate I
[0042] The raw material valdecoxib III (10.0 kg) was added to propionic anhydride (37.2 kg), stirred and dissolved. Add sulfamic acid (0.31kg), heat up to 50-60°C, and react for about 4 hours. TLC monitors that the reaction is complete, cools down to about 0°C, and keeps stirring for about 1 hour. After centrifugation, the filter cake was rinsed with methyl tert-butyl ether (20L) and placed in a vacuum oven at -0.08MPa~-0.1MPa, and dried at 50°C for 8-12 hours to obtain the parecoxib sodium intermediate (I) 9.31kg, yield 79.2%. The HPLC purity is 99.92%, the maximum single impurity is 0.05%, and the raw material valdecoxib III is <0.02%.
Embodiment 3
[0043] Embodiment 3: the preparation method of parecoxib sodium intermediate I
[0044] The raw material valdecoxib III (5.0 g) was added to propionic anhydride (18.6 g), and stirred to dissolve. Add methanesulfonic acid (0.15 g), heat up to 50-60° C., and react for about 3 hours. TLC monitors that the reaction is complete, cools down to about 0°C, and keeps stirring for about 1 hour. Filtrate, rinse the filter cake with methyl tert-butyl ether (10ml) and place it in a vacuum oven at -0.08MPa~-0.1MPa, and dry it in vacuum at 50°C for 8-12 hours to obtain 4.80g of parecoxib sodium intermediate 1, yield 81.5%. The HPLC purity is 99.90%, the maximum impurity is 0.07%, and the raw material valdecoxib III is <0.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com